Influence of cell type specific infectivity and tissue composition on SARS-CoV-2 infection dynamics within human airway epithelium
- PMID: 37566610
- PMCID: PMC10446191
- DOI: 10.1371/journal.pcbi.1011356
Influence of cell type specific infectivity and tissue composition on SARS-CoV-2 infection dynamics within human airway epithelium
Abstract
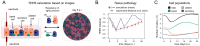
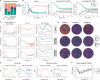
Human airway epithelium (HAE) represents the primary site of viral infection for SARS-CoV-2. Comprising different cell populations, a lot of research has been aimed at deciphering the major cell types and infection dynamics that determine disease progression and severity. However, the cell type-specific replication kinetics, as well as the contribution of cellular composition of the respiratory epithelium to infection and pathology are still not fully understood. Although experimental advances, including Air-liquid interface (ALI) cultures of reconstituted pseudostratified HAE, as well as lung organoid systems, allow the observation of infection dynamics under physiological conditions in unprecedented level of detail, disentangling and quantifying the contribution of individual processes and cells to these dynamics remains challenging. Here, we present how a combination of experimental data and mathematical modelling can be used to infer and address the influence of cell type specific infectivity and tissue composition on SARS-CoV-2 infection dynamics. Using a stepwise approach that integrates various experimental data on HAE culture systems with regard to tissue differentiation and infection dynamics, we develop an individual cell-based model that enables investigation of infection and regeneration dynamics within pseudostratified HAE. In addition, we present a novel method to quantify tissue integrity based on image data related to the standard measures of transepithelial electrical resistance measurements. Our analysis provides a first aim of quantitatively assessing cell type specific infection kinetics and shows how tissue composition and changes in regeneration capacity, as e.g. in smokers, can influence disease progression and pathology. Furthermore, we identified key measurements that still need to be assessed in order to improve inference of cell type specific infection kinetics and disease progression. Our approach provides a method that, in combination with additional experimental data, can be used to disentangle the complex dynamics of viral infection and immunity within human airway epithelial culture systems.
Copyright: © 2023 Raach et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium.mBio. 2020 Nov 6;11(6):e02852-20. doi: 10.1128/mBio.02852-20. mBio. 2020. PMID: 33158999 Free PMC article.
-
Cell Culture Differentiation and Proliferation Conditions Influence the In Vitro Regeneration of the Human Airway Epithelium.Am J Respir Cell Mol Biol. 2024 Sep;71(3):267-281. doi: 10.1165/rcmb.2023-0356MA. Am J Respir Cell Mol Biol. 2024. PMID: 38843491 Free PMC article.
-
The interplay between the airway epithelium and tissue macrophages during the SARS-CoV-2 infection.Front Immunol. 2022 Oct 6;13:991991. doi: 10.3389/fimmu.2022.991991. eCollection 2022. Front Immunol. 2022. PMID: 36275746 Free PMC article.
-
Long-term differentiating primary human airway epithelial cell cultures: how far are we?Cell Commun Signal. 2021 May 27;19(1):63. doi: 10.1186/s12964-021-00740-z. Cell Commun Signal. 2021. PMID: 34044844 Free PMC article. Review.
-
Air-liquid interface (ALI) impact on different respiratory cell cultures.Eur J Pharm Biopharm. 2023 Mar;184:62-82. doi: 10.1016/j.ejpb.2023.01.013. Epub 2023 Jan 22. Eur J Pharm Biopharm. 2023. PMID: 36696943 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

