Temporal scaling of dopamine neuron firing and dopamine release by distinct ion channels shape behavior
- PMID: 37566654
- PMCID: PMC10421029
- DOI: 10.1126/sciadv.adg8869
Temporal scaling of dopamine neuron firing and dopamine release by distinct ion channels shape behavior
Abstract
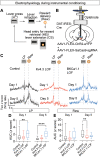
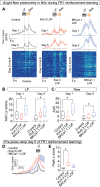
Dopamine is broadly implicated in reinforcement learning, but how patterns of dopamine activity are generated is poorly resolved. Here, we demonstrate that two ion channels, Kv4.3 and BKCa1.1, regulate the pattern of dopamine neuron firing and dopamine release on different time scales to influence separate phases of reinforced behavior in mice. Inactivation of Kv4.3 in VTA dopamine neurons increases ex vivo pacemaker activity and excitability that is associated with increased in vivo firing rate and ramping dynamics before lever press in a learned instrumental paradigm. Loss of Kv4.3 enhances performance of the learned response and facilitates extinction. In contrast, loss of BKCa1.1 increases burst firing and phasic dopamine release that enhances learning of an instrumental response and enhances extinction burst lever pressing in early extinction that is associated with a greater change in activity between reinforced and unreinforced actions. These data demonstrate that disruption of intrinsic regulators of neuronal activity differentially affects dopamine dynamics during reinforcement and extinction learning.
Figures




References
MeSH terms
Substances
Grants and funding
- R01 MH104450/MH/NIMH NIH HHS/United States
- P30 DA048736/DA/NIDA NIH HHS/United States
- S10 OD016240/OD/NIH HHS/United States
- F32 MH127801/MH/NIMH NIH HHS/United States
- F31 MH116549/MH/NIMH NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- F31 DA053724/DA/NIDA NIH HHS/United States
- T32 HL007028/HL/NHLBI NIH HHS/United States
- K99 DA054265/DA/NIDA NIH HHS/United States
- F31 MH126489/MH/NIMH NIH HHS/United States
- R01 DA044315/DA/NIDA NIH HHS/United States
- R03 TR003307/TR/NCATS NIH HHS/United States
- R00 DA054265/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

