Development of a Dihydroquinoline-Pyrazoline GluN2C/2D-Selective Negative Allosteric Modulator of the N-Methyl-d-aspartate Receptor
- PMID: 37566734
- PMCID: PMC10485906
- DOI: 10.1021/acschemneuro.3c00181
Development of a Dihydroquinoline-Pyrazoline GluN2C/2D-Selective Negative Allosteric Modulator of the N-Methyl-d-aspartate Receptor
Abstract
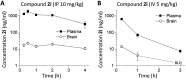
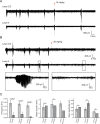
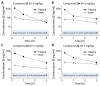
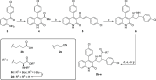
Subunit-selective inhibition of N-methyl-d-aspartate receptors (NMDARs) is a promising therapeutic strategy for several neurological disorders, including epilepsy, Alzheimer's and Parkinson's disease, depression, and acute brain injury. We previously described the dihydroquinoline-pyrazoline (DQP) analogue 2a (DQP-26) as a potent NMDAR negative allosteric modulator with selectivity for GluN2C/D over GluN2A/B. However, moderate (<100-fold) subunit selectivity, inadequate cell-membrane permeability, and poor brain penetration complicated the use of 2a as an in vivo probe. In an effort to improve selectivity and the pharmacokinetic profile of the series, we performed additional structure-activity relationship studies of the succinate side chain and investigated the use of prodrugs to mask the pendant carboxylic acid. These efforts led to discovery of the analogue (S)-(-)-2i, also referred to as (S)-(-)-DQP-997-74, which exhibits >100- and >300-fold selectivity for GluN2C- and GluN2D-containing NMDARs (IC50 0.069 and 0.035 μM, respectively) compared to GluN2A- and GluN2B-containing receptors (IC50 5.2 and 16 μM, respectively) and has no effects on AMPA, kainate, or GluN1/GluN3 receptors. Compound (S)-(-)-2i is 5-fold more potent than (S)-2a. In addition, compound 2i shows a time-dependent enhancement of inhibitory actions at GluN2C- and GluN2D-containing NMDARs in the presence of the agonist glutamate, which could attenuate hypersynchronous activity driven by high-frequency excitatory synaptic transmission. Consistent with this finding, compound 2i significantly reduced the number of epileptic events in a murine model of tuberous sclerosis complex (TSC)-induced epilepsy that is associated with upregulation of the GluN2C subunit. Thus, 2i represents a robust tool for the GluN2C/D target validation. Esterification of the succinate carboxylate improved brain penetration, suggesting a strategy for therapeutic development of this series for NMDAR-associated neurological conditions.
Keywords: NR2C; NR2D; blood–brain barrier; epilepsy; seizure; tuberous sclerosis complex.
Conflict of interest statement
The authors declare the following competing financial interest(s): S.F.T. is a member of the medical advisory boards for the CureGRIN Foundation and the GRIN2B Foundation, is a member of the scientific advisory boards for Sage Therapeutics and Eumentis Therapeutics, is a Senior Advisor for GRIN Therapeutics, is a consultant for Neurocrine, is a cofounder of NeurOp, Inc. and AgriThera, Inc., and is on the Board of Directors for NeurOp Inc. D.C.L. is on the Board of Directors for NeurOp Inc. Multiple authors are coinventors on Emory-owned IP involving NMDA receptor modulators (M.P.D., P.M., Y.J., D.S.M., P.J.A., R.G.F., N.S.A., H.Y., S.F.T., and D.C.L.). H.Y. is PI on a grant from Sage Therapeutics to Emory. K.C. is an employee of Janssen Research and Development. The other authors declare no competing financial interest.
Figures

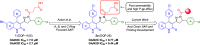

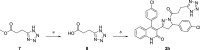

Similar articles
-
A Novel Negative Allosteric Modulator Selective for GluN2C/2D-Containing NMDA Receptors Inhibits Synaptic Transmission in Hippocampal Interneurons.ACS Chem Neurosci. 2018 Feb 21;9(2):306-319. doi: 10.1021/acschemneuro.7b00329. Epub 2017 Nov 2. ACS Chem Neurosci. 2018. PMID: 29043770 Free PMC article.
-
Distinct GluN1 and GluN2 Structural Determinants for Subunit-Selective Positive Allosteric Modulation of N-Methyl-d-aspartate Receptors.ACS Chem Neurosci. 2021 Jan 6;12(1):79-98. doi: 10.1021/acschemneuro.0c00561. Epub 2020 Dec 16. ACS Chem Neurosci. 2021. PMID: 33326224 Free PMC article.
-
Structure-activity relationships and pharmacophore model of a noncompetitive pyrazoline containing class of GluN2C/GluN2D selective antagonists.J Med Chem. 2013 Aug 22;56(16):6434-56. doi: 10.1021/jm400652r. Epub 2013 Aug 2. J Med Chem. 2013. PMID: 23909910 Free PMC article.
-
Investigation of the structural requirements for N-methyl-D-aspartate receptor positive and negative allosteric modulators based on 2-naphthoic acid.Eur J Med Chem. 2019 Feb 15;164:471-498. doi: 10.1016/j.ejmech.2018.12.054. Epub 2018 Dec 28. Eur J Med Chem. 2019. PMID: 30622023 Free PMC article. Review.
-
Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators.Neurochem Int. 2012 Sep;61(4):581-92. doi: 10.1016/j.neuint.2012.01.004. Epub 2012 Jan 17. Neurochem Int. 2012. PMID: 22269804 Free PMC article. Review.
Cited by
-
Structural basis for channel gating and blockade in tri-heteromeric GluN1-2B-2D NMDA receptor.Neuron. 2025 Apr 2;113(7):991-1005.e5. doi: 10.1016/j.neuron.2025.01.013. Epub 2025 Feb 14. Neuron. 2025. PMID: 39954679
References
-
- Hansen K. B.; Wollmuth L. P.; Bowie D.; Furukawa H.; Menniti F. S.; Sobolevsky A. I.; Swanson G. T.; Swanger S. A.; Greger I. H.; Nakagawa T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73 (4), 298–487. 10.1124/pharmrev.120.000131. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

