Optogenetic targeting of astrocytes restores slow brain rhythm function and slows Alzheimer's disease pathology
- PMID: 37567942
- PMCID: PMC10421876
- DOI: 10.1038/s41598-023-40402-3
Optogenetic targeting of astrocytes restores slow brain rhythm function and slows Alzheimer's disease pathology
Abstract
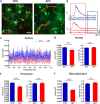
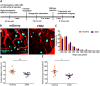
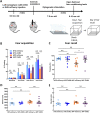
Patients with Alzheimer's disease (AD) exhibit non-rapid eye movement (NREM) sleep disturbances in addition to memory deficits. Disruption of NREM slow waves occurs early in the disease progression and is recapitulated in transgenic mouse models of beta-amyloidosis. However, the mechanisms underlying slow-wave disruptions remain unknown. Because astrocytes contribute to slow-wave activity, we used multiphoton microscopy and optogenetics to investigate whether they contribute to slow-wave disruptions in APP/PS1 mice. The power but not the frequency of astrocytic calcium transients was reduced in APP/PS1 mice compared to nontransgenic controls. Optogenetic activation of astrocytes at the endogenous frequency of slow waves restored slow-wave power, reduced amyloid deposition, prevented neuronal calcium elevations, and improved memory performance. Our findings revealed malfunction of the astrocytic network driving slow-wave disruptions. Thus, targeting astrocytes to restore circuit activity underlying sleep and memory disruptions in AD could ameliorate disease progression.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Optogenetic Targeting of Astrocytes Restores Slow Brain Rhythm Function and Slows Alzheimer's Disease Pathology.Res Sq [Preprint]. 2023 Apr 25:rs.3.rs-2813056. doi: 10.21203/rs.3.rs-2813056/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Aug 11;13(1):13075. doi: 10.1038/s41598-023-40402-3. PMID: 37163040 Free PMC article. Updated. Preprint.
References
-
- Bloom GS. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. - PubMed
-
- Calderon-Garcidueñas AL, Duyckaerts C. Chapter 23 - Alzheimer disease. In: Kovacs GG, Alafuzoff I, editors. Handbook of Clinical Neurology. Elsevier; 2018. pp. 325–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

