Novel expression system based on enhanced permeability of Vibrio natriegens cells induced by D,D- carboxypeptidase overexpression
- PMID: 37568013
- PMCID: PMC10421817
- DOI: 10.1007/s11274-023-03723-z
Novel expression system based on enhanced permeability of Vibrio natriegens cells induced by D,D- carboxypeptidase overexpression
Abstract
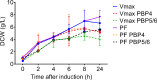
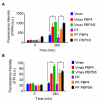
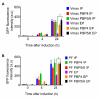
Vibrio natriegens is a fast-growing, non-pathogenic marine bacterium with promising features for biotechnological applications such as high-level recombinant protein production or fast DNA propagation. A remarkable short generation time (< 10 min), robust proteosynthetic activity and versatile metabolism with abilities to utilise wide range of substrates contribute to its establishment as a future industrial platform for fermentation processes operating with high productivity.D,D-carboxypeptidases are membrane-associated enzymes involved in peptidoglycan biosynthesis and cell wall formation. This study investigates the impact of overexpressed D,D-carboxypeptidases on membrane integrity and the increased leakage of intracellular proteins into the growth medium in V. natriegens. Our findings confirm that co-expression of these enzymes can enhance membrane permeability, thereby facilitating the transport of target proteins into the extracellular environment, without the need for secretion signals, tags, or additional permeabilization methods. Using only a single step IMAC chromatography, we were able to purify AfKatG, MDBP or Taq polymerase in total yields of 117.9 ± 56.0 mg/L, 36.5 ± 12.9 mg/L and 26.5 ± 6.0 mg/L directly from growth medium, respectively. These results demonstrate the feasibility of our V. natriegens based system as a broadly applicable extracellular tag-less recombinant protein producer.
Keywords: D,D-carboxypeptidases; Extracellular production; Recombinant proteins; Vibrio natriegens.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
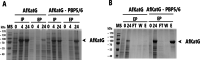


References
-
- Denome SA, Elf PK, Henderson TA, Nelson DE, Young KD. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol. 1999;181(13):3981–3993. doi: 10.1128/JB.181.13.3981-3993.1999. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

