Acid ceramidase gene therapy ameliorates pulmonary arterial hypertension with right heart dysfunction
- PMID: 37568148
- PMCID: PMC10416391
- DOI: 10.1186/s12931-023-02487-2
Acid ceramidase gene therapy ameliorates pulmonary arterial hypertension with right heart dysfunction
Abstract
Background: Up-regulation of ceramides in pulmonary hypertension (PH), contributing to perturbations in sphingolipid homeostasis and the transition of cells to a senescence state. We assessed the safety, feasibility, and efficiency of acid ceramidase gene transfer in a rodent PH model.
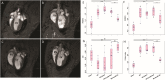
Methods: A model of PH was established by the combination of left pneumonectomy and injection of Sugen toxin. Magnetic resonance imaging and right heart catheterization confirmed development of PH. Animals were subjected to intratracheal administration of synthetic adeno-associated viral vector (Anc80L65) carrying the acid ceramidase (Anc80L65.AC), an empty capsid vector, or saline. Therapeutic efficacy was evaluated 8 weeks after gene delivery.
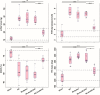
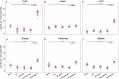
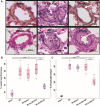
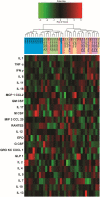
Results: Hemodynamic assessment 4 weeks after PH model the development demonstrated an increase in the mean pulmonary artery pressure to 30.4 ± 2.13 mmHg versus 10.4 ± 1.65 mmHg in sham (p < 0.001), which was consistent with the definition of PH. We documented a significant increase in pulmonary vascular resistance in the saline-treated (6.79 ± 0.85 mm Hg) and empty capsid (6.94 ± 0.47 mm Hg) groups, but not in animals receiving Anc80L65.AC (4.44 ± 0.71 mm Hg, p < 0.001). Morphometric analysis demonstrated an increase in medial wall thickness in control groups in comparison to those treated with acid ceramidase. After acid ceramidase gene delivery, a significant decrease of pro-inflammatory factors, interleukins, and senescence markers was observed.
Conclusion: Gene delivery of acid ceramidase provided tropism to pulmonary tissue and ameliorated vascular remodeling with right ventricular dysfunction in pulmonary hypertension.
Keywords: Adeno-associated virus; And acid ceramidase; Gene therapy; Pulmonary arterial hypertension; Sphingolipid metabolism.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Intratracheal Gene Delivery of SERCA2a Ameliorates Chronic Post-Capillary Pulmonary Hypertension: A Large Animal Model.J Am Coll Cardiol. 2016 May 3;67(17):2032-46. doi: 10.1016/j.jacc.2016.02.049. J Am Coll Cardiol. 2016. PMID: 27126531 Free PMC article.
-
Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction.Circulation. 2018 Apr 24;137(17):1796-1810. doi: 10.1161/CIRCULATIONAHA.117.031608. Epub 2017 Dec 15. Circulation. 2018. PMID: 29246894 Free PMC article.
-
Right Ventricular and Right Atrial Function Are Less Compromised in Pulmonary Hypertension Secondary to Heart Failure With Preserved Ejection Fraction: A Comparison With Pulmonary Arterial Hypertension With Similar Pressure Overload.Circ Heart Fail. 2022 Feb;15(2):e008726. doi: 10.1161/CIRCHEARTFAILURE.121.008726. Epub 2021 Dec 23. Circ Heart Fail. 2022. PMID: 34937392 Free PMC article.
-
Molecular Pathways in Pulmonary Arterial Hypertension.Int J Mol Sci. 2022 Sep 2;23(17):10001. doi: 10.3390/ijms231710001. Int J Mol Sci. 2022. PMID: 36077398 Free PMC article. Review.
-
Pulmonary Hypertension Due to Left Heart Disease: Diagnosis, Pathophysiology, and Therapy.Hypertension. 2020 Jun;75(6):1397-1408. doi: 10.1161/HYPERTENSIONAHA.119.14330. Epub 2020 Apr 27. Hypertension. 2020. PMID: 32336230 Review.
Cited by
-
Metabolic gene therapy in a canine with pulmonary hypertension secondary to degenerative mitral valve disease.Front Vet Sci. 2024 Sep 23;11:1415030. doi: 10.3389/fvets.2024.1415030. eCollection 2024. Front Vet Sci. 2024. PMID: 39376911 Free PMC article.
-
From multi-omics approaches to personalized medicine in myocardial infarction.Front Cardiovasc Med. 2023 Oct 30;10:1250340. doi: 10.3389/fcvm.2023.1250340. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37965091 Free PMC article. Review.
References
-
- Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, Potus F, Omura J, Cook T, Fisher A, Rodriguez B, Brown RD, Stenmark KR, Rubinstein CD, Krentz K, Tabima DM, Li R, Sun X, Chesler NC, Provencher S, Bonnet S, Lahm T. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest. 2021;131(6):e129433. doi: 10.1172/JCI129433. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

