Fetal Muse-based therapy prevents lethal radio-induced gastrointestinal syndrome by intestinal regeneration
- PMID: 37568164
- PMCID: PMC10416451
- DOI: 10.1186/s13287-023-03425-1
Fetal Muse-based therapy prevents lethal radio-induced gastrointestinal syndrome by intestinal regeneration
Abstract
Background: Human multilineage-differentiating stress enduring (Muse) cells are nontumorigenic endogenous pluripotent-like stem cells that can be easily obtained from various adult or fetal tissues. Regenerative effects of Muse cells have been shown in some disease models. Muse cells specifically home in damaged tissues where they exert pleiotropic effects. Exposition of the small intestine to high doses of irradiation (IR) delivered after radiotherapy or nuclear accident results in a lethal gastrointestinal syndrome (GIS) characterized by acute loss of intestinal stem cells, impaired epithelial regeneration and subsequent loss of the mucosal barrier resulting in sepsis and death. To date, there is no effective medical treatment for GIS. Here, we investigate whether Muse cells can prevent lethal GIS and study how they act on intestinal stem cell microenvironment to promote intestinal regeneration.
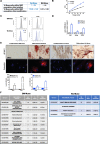
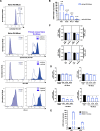
Methods: Human Muse cells from Wharton's jelly matrix of umbilical cord (WJ-Muse) were sorted by flow cytometry using the SSEA-3 marker, characterized and compared to bone-marrow derived Muse cells (BM-Muse). Under gas anesthesia, GIS mice were treated or not through an intravenous retro-orbital injection of 50,000 WJ-Muse, freshly isolated or cryopreserved, shortly after an 18 Gy-abdominal IR. No immunosuppressant was delivered to the mice. Mice were euthanized either 24 h post-IR to assess early small intestine tissue response, or 7 days post-IR to assess any regenerative response. Mouse survival, histological stainings, apoptosis and cell proliferation were studied and measurement of cytokines, recruitment of immune cells and barrier functional assay were performed.
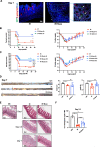
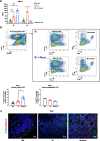
Results: Injection of WJ-Muse shortly after abdominal IR highly improved mouse survival as a result of a rapid regeneration of intestinal epithelium with the rescue of the impaired epithelial barrier. In small intestine of Muse-treated mice, an early enhanced secretion of IL-6 and MCP-1 cytokines was observed associated with (1) recruitment of monocytes/M2-like macrophages and (2) proliferation of Paneth cells through activation of the IL-6/Stat3 pathway.
Conclusion: Our findings indicate that a single injection of a small quantity of WJ-Muse may be a new and easy therapeutic strategy for treating lethal GIS.
Keywords: Muse cells; Radio-induced gastro-intestinal syndrome; Regeneration; Stem cell microenvironment.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Gelberg HB. Comparative anatomy, physiology, and mechanisms of disease production of the esophagus, stomach, and small intestine. Toxicol Pathol. 2014;42:54–66. - PubMed
-
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

