Paternal Age Matters: Association with Sperm Criteria's- Spermatozoa DNA Integrity and Methylation Profile
- PMID: 37568329
- PMCID: PMC10420110
- DOI: 10.3390/jcm12154928
Paternal Age Matters: Association with Sperm Criteria's- Spermatozoa DNA Integrity and Methylation Profile
Abstract
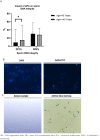
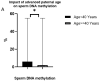
Advanced age has been reported to negatively affect sperm parameters and spermatozoa DNA integrity. A decline in sperm criteria was also associated with altered epigenetic marks such as DNA methylation with a potential downstream impact on in vitro fertilization success and clinical outcomes. The aim of the present retrospective study was to clarify the association between advanced paternal age (APA) and sperm parameters, DNA integrity and DNA methylation profile. A total of 671 patients consulting for infertility underwent sperm analysis, sperm DNA integrity assessment and methylation level measurement. The principal finding was that individuals over 40 years of age exhibit a significant increase in DNA fragmentation levels compared to the younger group (15% versus 9%, respectively, p = 0.04). However, there was no significant difference in DNA decondensation and sperm parameters in association with APA. In addition, a drop in the global methylation level was also found in men over 40 years (6% in the young group versus 2% in the old group, p = 0.03). As a conclusion, men over 40 years are at higher risk of elevated sperm DNA fragmentation and lower methylation level. Based on these observations, it is recommended that the assessment of sperm DNA fragmentation should be taken into consideration particularly after the age of 40. Our findings support the idea that paternal age is a crucial factor that should not be neglected during fertility evaluation and treatment since it is associated with epigenetics changes in sperm. Although the underlying mechanism remains to be clarified, we believe that environmental and professional exposure factors are likely involved in the process.
Keywords: paternal age; sperm parameters; spermatozoa DNA integrity and methylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bahri H., Ben Khalifa M., Ben Rhouma M., Abidi Z., Abbassi E., Ben Rhouma K., Benkhalifa M. Decline in semen quality of North Afri-can men: A retrospective study of 20,958 sperm analyses of men from different North African countries tested in Tunisia over a period of 6 years (2013–2018) Ann. Hum. Biol. 2021;48:350–359. doi: 10.1080/03014460.2021.1957501. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

