Hypertensive Heart Failure
- PMID: 37568493
- PMCID: PMC10419453
- DOI: 10.3390/jcm12155090
Hypertensive Heart Failure
Abstract
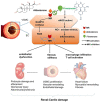
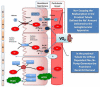
Despite overwhelming epidemiological evidence, the contribution of hypertension (HTN) to heart failure (HF) development has been undermined in current clinical practice. This is because approximately half of HF patients have been labeled as suffering from HF with preserved left ventricular (LV) ejection fraction (EF) (HFpEF), with HTN, obesity, and diabetes mellitus (DM) being considered virtually equally responsible for its development. However, this suggestion is obviously inaccurate, since HTN is by far the most frequent and devastating morbidity present in HFpEF. Further, HF development in obesity or DM is rare in the absence of HTN or coronary artery disease (CAD), whereas HTN often causes HF per se. Finally, unlike HTN, for most major comorbidities present in HFpEF, including anemia, chronic kidney disease, pulmonary disease, DM, atrial fibrillation, sleep apnea, and depression, it is unknown whether they precede HF or result from it. The purpose of this paper is to provide a contemporary overview on hypertensive HF, with a special emphasis on its inflammatory nature and association with autonomic nervous system (ANS) imbalance, since both are of pathophysiologic and therapeutic interest.
Keywords: autonomic imbalance; ejection fraction; heart failure; hypertension; sympathetic nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Conrad N., Judge A., Tran J., Mohseni H., Hedgecott D., Crespillo A.P., Allison M., Hemingway H., Cleland J.G., McMurray J.J.V., et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet. 2018;391:572–580. doi: 10.1016/S0140-6736(17)32520-5. - DOI - PMC - PubMed
-
- Rapsomaniki E., Timmis A., George J., Pujades-Rodriguez M., Shah A.D., Denaxas S., White I.R., Caulfield M.J., Deanfield J.E., Smeeth L., et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

