Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma
- PMID: 37568605
- PMCID: PMC10417036
- DOI: 10.3390/cancers15153789
Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma
Abstract
Background: The aim of this study was to evaluate the clinical impact of a combination of systemic sequential therapy and locoregional therapy on the long-term survival of patients with Barcelona Clinic Liver Cancer (BCLC) stage C hepatocellular carcinoma (HCC).
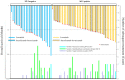
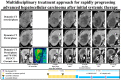
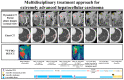
Methods: Sixty-four consecutive patients with intrahepatic target nodules who had initially received systemic therapy (lenvatinib and atezolizumab plus bevacizumab) were reviewed. The clinical impact of the combined use of systemic sequential therapy and locoregional therapy was evaluated by determining overall survival (OS). The combined use of systemic sequential therapy with more than two agents and locoregional treatment was defined as multidisciplinary combination therapy (MCT), while only systemic sequential therapy and repeated locoregional-treatment was defined as a single treatment procedure (STP).
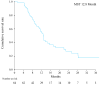
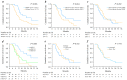
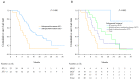
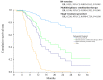
Results: R0 resection, MCT, and STP resulted in significantly better OS compared with no additional treatment (median OS, not reached vs. 18.2 months and 12.6 vs. 8.1 months, respectively; p = 0.002). Multivariate analysis confirmed that the use of R0 resection and MCT were associated with better OS (hazard ratio [HR]; 0.053, p = 0.006 and 0.189, p < 0.001, respectively) compared with that for STP (HR; 0.279, p = 0.003).
Conclusions: MCT is may effective in patients with BCLC stage C HCC and intrahepatic target nodules who have previously received systemic therapy-based treatment.
Keywords: atezolizumab plus bevacizumab; combination therapy; hepatocellular carcinoma; lenvatinib; locoregional treatment; systemic therapy.
Conflict of interest statement
Yusuke Kawamura reports honoraria from Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd., and TERUMO CORPORATION. Junichi Shindoh reports honoraria from Eisai Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Hiromitsu Kumada reports honoraria from Eisai Co., Ltd. The other authors declare no conflict of interest.
Figures

References
-
- Reig M., Forner A., Rimola J., Ferrer-Fabrega J., Burrel M., Garcia-Criado A., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

