The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth
- PMID: 37568667
- PMCID: PMC10416835
- DOI: 10.3390/cancers15153851
The Bivalent Bromodomain Inhibitor MT-1 Inhibits Prostate Cancer Growth
Abstract
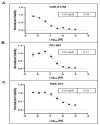
Bromodomains (BD) are epigenetic readers of histone acetylation involved in chromatin remodeling and transcriptional regulation of several genes including protooncogene cellular myelocytomatosis (c-Myc). c-Myc is difficult to target directly by agents due to its disordered alpha helical protein structure and predominant nuclear localization. The epigenetic targeting of c-Myc by BD inhibitors is an attractive therapeutic strategy for prostate cancer (PC) associated with increased c-Myc upregulation with advancing disease. MT-1 is a bivalent BD inhibitor that is 100-fold more potent than the first-in-class BD inhibitor JQ1. MT-1 decreased cell viability and causes cell cycle arrest in G0/G1 phase in castration-sensitive and resistant PC cell lines in a dose-dependent fashion. The inhibition of c-Myc function by MT-1 was molecularly corroborated by the de-repression of Protein Kinase D1 (PrKD) and increased phosphorylation of PrKD substrate proteins: threonine 120, serine 11, and serine 216 amino acid residues in β-Catenin, snail, and cell division cycle 25c (CDC25c) proteins, respectively. The treatment of 3D cell cultures derived from three unique clinically annotated heavily pretreated patient-derived PC xenografts (PDX) mice models with increasing doses of MT-1 demonstrated the lowest IC50 in tumors with c-Myc amplification and clinically resistant to Docetaxel, Cabazitaxel, Abiraterone, and Enzalutamide. An intraperitoneal injection of either MT-1 or in combination with 3jc48-3, an inhibitor of obligate heterodimerization with MYC-associated protein X (MAX), in mice implanted with orthotopic PC PDX, decreased tumor growth. This is the first pre-clinical study demonstrating potential utility of MT-1 in the treatment of PC with c-Myc dysregulation.
Keywords: MT-1; bromodomain inhibitors; c-Myc; patient-derived xenografts; prostate cancer.
Conflict of interest statement
The authors have no potential, perceived, or real conflict of interest in relation to this research project or manuscript.
Figures



References
-
- Kalkat M., Resetca D., Lourenco C., Chan P.-K., Wei Y., Shiah Y.-J., Vitkin N., Tong Y., Sunnerhagen M., Done S.J., et al. MYC Protein Interactome Profiling Reveals Functionally Distinct Regions that Cooperate to Drive Tumorigenesis. Mol. Cell. 2018;72:836–848.e7. doi: 10.1016/j.molcel.2018.09.031. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

