MicroRNA-371a-3p-The Novel Serum Biomarker in Testicular Germ Cell Tumors
- PMID: 37568759
- PMCID: PMC10417034
- DOI: 10.3390/cancers15153944
MicroRNA-371a-3p-The Novel Serum Biomarker in Testicular Germ Cell Tumors
Abstract
Introduction: Testicular germ cell tumors (TGCTs) are a paradigm for the use of serum tumor markers in clinical management. However, conventional markers such as alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (hCG), and lactate dehydrogenase (LDH) have quite limited sensitivities and specificities. Within the last decade, the microRNA-371a-3p (miR371) emerged as a possible new biomarker with promising features.
Areas covered: This review covers the typical features as well as possible clinical applications of miR371 in TGCT patients, such as initial diagnosis, therapy monitoring, and follow-up. Additionally, technical issues are discussed.
Expert opinion: With a sensitivity of around 90% and specificity >90%, miR371 clearly outperforms the classical serum tumor markers in TGCTs. The unique features of the test involve the potential of modifying recent standards of care in TGCT. In particular, miR371 is expected to aid clinical decision-making in scenarios such as discriminating small testicular TGCT masses from benign ones prior to surgery, assessing equivocal lymphadenopathies, and monitoring chemotherapy results. Likewise, it is expected to make follow-up easier by reducing the intensity of examinations and by sparing imaging procedures. Overall, the data presently available are promising, but further prospective studies are required before the test can be implemented in standard clinical care.
Keywords: MicroRNA; biomarker; germ cell tumor; miRNA; testicular cancer.
Conflict of interest statement
T.N. and J.S. have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. K.-P.D. and G.B. declare ownership shares of each 8.91% of mirdetect GmbH, Bremerhaven, Germany.
Figures
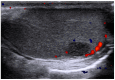

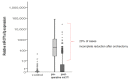
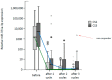

References
-
- Huang J., Chan S.C., Tin M.S., Liu X., Lok V.T., Ngai C.H., Zhang L., Lucero-Prisno D.E.R., Xu W., Zheng Z.J., et al. Worldwide Distribution, Risk Factors, and Temporal Trends of Testicular Cancer Incidence and Mortality: A Global Analysis. Eur. Urol. Oncol. 2022;5:566–576. doi: 10.1016/j.euo.2022.06.009. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

