Cell State and Cell Type: Deconvoluting Circulating Tumor Cell Populations in Liquid Biopsies by Multi-Omics
- PMID: 37568766
- PMCID: PMC10417732
- DOI: 10.3390/cancers15153949
Cell State and Cell Type: Deconvoluting Circulating Tumor Cell Populations in Liquid Biopsies by Multi-Omics
Abstract
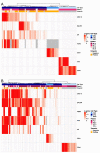
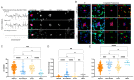
Bi-directional crosstalk between the tumor and the tumor microenvironment (TME) has been shown to increase the rate of tumor evolution and to play a key role in neoplastic progression, therapeutic resistance, and a patient's overall survival. Here, we set out to use a comprehensive liquid-biopsy analysis to study cancer and specific TME cells in circulation and their association with disease status. Cytokeratin+, CD45- circulating rare cells (CRCs) from nine breast and four prostate cancer patients were characterized through morphometrics, single-cell copy number analysis, and targeted multiplexed proteomics to delineate cancer cell lineage from other rare cells originating in the TME. We show that we can detect epithelial circulating tumor cells (EPI.CTC), CTCs undergoing epithelial-to-mesenchymal transition (EMT.CTC) and circulating endothelial cells (CECs) using a universal rare event detection platform (HDSCA). Longitudinal analysis of an index patient finds that CTCs are present at the time of disease progression, while CECs are predominately present at the time of stable disease. In a small cohort of prostate and breast cancer patients, we find high inter-patient and temporal intra-patient variability in the expression of tissue specific markers such as ER, HER2, AR, PSA and PSMA and EpCAM. Our study stresses the importance of the multi-omic characterization of circulating rare cells in patients with breast and prostate carcinomas, specifically highlighting overlapping and cell type defining proteo-genomic characteristics of CTCs and CECs.
Keywords: breast cancer; circulating endothelial cells; circulating tumor cells; epithelial–mesenchymal transition; liquid biopsy; prostate cancer.
Conflict of interest statement
P.K. and J.H. hold ownership interest in, are consultants to and receive royalties from Epic Sciences for licensed technology. A.K. is a shareholder in Epic Sciences. Epic Sciences and the University of Southern California, USC Michelson Center (P.K. and J.H.), have signed a sponsored research agreement to advance next-generation liquid biopsy technology for precision oncology. C.R.V. is a royalty recipient for technology licensed to Epic Sciences. P.S. has received grants (payments made to the institution) from Novartis, Bristol Myers Squibb, Merck, and Gilead; royalties from UpToDate; and advisory board participation for Pfizer, Merck, Gilead, Genzyme Corporation, Novartis, AstraZeneca, GSK, and Seattle Genetics. H.C.F.M. has received consulting fees from Myovant Sciences and has received research funding (payments made to her institution) from AstraZeneca, Daiichi-Sankyo, CytoDyn, Inc., Roche-Genentech, Sermonix Pharmaceuticals and Seattle Genetics. All other authors have no conflict to report.
Figures




References
-
- Bremnes R.M., Dønnem T., Al-Saad S., Al-Shibli K., Andersen S., Sirera R., Camps C., Marinez I., Busund L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. - DOI - PubMed
-
- Chai S., Matsumoto N., Storgard R., Peng C.C., Aparicio A., Ormseth B., Rappard K., Cunningham K., Kolatkar A., Nevarez R., et al. Platelet-Coated Circulating Tumor Cells Are a Predictive Biomarker in Patients with Metastatic Castrate-Resistant Prostate Cancer. Mol. Cancer Res. 2021;19:2036–2045. doi: 10.1158/1541-7786.MCR-21-0383. - DOI - PubMed
-
- Rossi G., Mu Z., Rademaker A.W., Austin L.K., Strickland K.S., Costa R.L.B., Nagy R.J., Zagonel V., Taxter T.J., Behdad A., et al. Cell-Free DNA and Circulating Tumor Cells: Comprehensive Liquid Biopsy Analysis in Advanced Breast Cancer. Clin. Cancer Res. 2018;24:560–568. doi: 10.1158/1078-0432.CCR-17-2092. - DOI - PubMed
Grants and funding
- U10 CA180821/CA/NCI NIH HHS/United States
- U24 CA196175/CA/NCI NIH HHS/United States
- U10CA180868/CA/NCI NIH HHS/United States
- P20GM13042/GM/NIGMS NIH HHS/United States
- U54CA143906/CA/NCI NIH HHS/United States
- P20 GM130423/GM/NIGMS NIH HHS/United States
- U24CA196175/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10CA180819/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U54 CA143906/CA/NCI NIH HHS/United States
- U10 CA180868/CA/NCI NIH HHS/United States
- U10CA180888/CA/NCI NIH HHS/United States
- U54 CA143907/CA/NCI NIH HHS/United States
- U10CA180821/CA/NCI NIH HHS/United States
- U54CA143907/CA/NCI NIH HHS/United States
- U10CA180820/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

