Microcephaly and Chorioretinopathy Relevance as a Differential Diagnosis
- PMID: 37568951
- PMCID: PMC10417591
- DOI: 10.3390/diagnostics13152588
Microcephaly and Chorioretinopathy Relevance as a Differential Diagnosis
Abstract
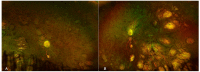
Microcephaly and chorioretinopathy are genetic disorders that are inherited in an autosomal recessive manner. The most frequent ocular manifestation is the presence of lacunar atrophy in the retina and choroid. The diagnosis of this condition can be challenging as several potential causes and related syndromes need to be ruled out. We present two cases of microcephaly and chorioretinopathy in Mexican patients, their clinical characterization, and discuss the differential diagnoses that should be considered. An 8-year-old girl was examined due to a history of decreased vision in both eyes. Fundus examination showed excavated, well-defined, sectorial, bilateral, and symmetrical areas of chorioretinal atrophy. An 18-year-old male had a history of poor vision since childhood. Previous ophthalmological examinations reported bilateral symmetric chorioretinal atrophy with pigment accumulation. Both patients had a prior diagnosis of microcephaly and language delay. Blood tests and a comprehensive systemic evaluation ruled out intrauterine infections. The electroretinogram showed decreased amplitude and increased implicit time in the photopic and scotopic responses. Genetic tests revealed mutations in the TUBGCP4 gene, leading to a diagnosis of microcephaly and chorioretinopathy. As observed in these cases, there was variability in retinal lesions. The presence of chorioretinal lacunae and genetic testing can help to correctly diagnose this disorder.
Keywords: chorioretinal atrophy; genetics of retinal diseases; inherited diseases; microcephaly and chorioretinopathy; retina; retinal degeneration.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Jones G.E., Ostergaard P., Moore A.T., Connell F.C., Williams D., Quarrell O., Brady A.F., Spier I., Hazan F., Moldovan O., et al. Microcephaly with or without chorioretinopathy, lymphoedema, or mental retardation (MCLMR): Review of phenotype associated with KIF11 mutations. Eur. J. Hum. Genet. 2014;22:881–887. doi: 10.1038/ejhg.2013.263. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

