Trypanosoma cruzi Secreted Cyclophilin Tc CyP19 as an Early Marker for Trypanocidal Treatment Efficiency
- PMID: 37569250
- PMCID: PMC10418876
- DOI: 10.3390/ijms241511875
Trypanosoma cruzi Secreted Cyclophilin Tc CyP19 as an Early Marker for Trypanocidal Treatment Efficiency
Abstract
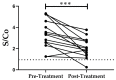
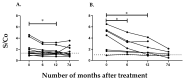
Cyclophilins (CyPs) are a family of enzymes involved in protein folding. Trypanosoma cruzi, the causative agent of Chagas disease, has a 19-kDa cyclophilin, TcCyP19, that was found to be secreted in parasite stages of the CL Brener clone and recognized by sera from T. cruzi-infected mice and patients. The levels of specific antibodies against TcCyP19 in T. cruzi-infected mice and subjects before and after drug treatment were measured by an in-house enzyme linked immunosorbent assay (ELISA). Mice in the acute and chronic phase of infection, with successful trypanocidal treatments, showed significantly lower anti-TcCyP19 antibody levels than untreated mice. In children and adults chronically infected with T. cruzi, a significant decrease in the anti-TcCyP19 titers was observed after 12 months of etiological treatment. This decrease was maintained in adult chronic patients followed-up 30-38 months post-treatment. These results encourage further studies on TcCyP19 as an early biomarker of trypanocidal treatment efficiency.
Keywords: Chagas; ELISA; TcCyP19; Trypanosoma cruzi; benznidazole; biomarker; cyclophilin; nifurtimox; parasiticidal treatment.
Conflict of interest statement
No potential conflicts of interest are disclosed by any authors, since this research was conducted in the absence of any commercial or financial relationships.
Figures



References
-
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391:82–94. - PubMed
-
- World Health Organization Chagas Disease (American Trypanosomiasis) [(accessed on 20 May 2023)]. Available online: https://www.who.int/health-topics/chagas-disease.
-
- Rassi A., Jr., Rassi A., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Lancet. 2012;26:275–291. - PubMed
-
- Zacks M.A., Wen J.J., Vyatkina G., Bhatia V., Garg N. An overview of chagasic cardiomyopathy: Pathogenic importance of oxidative stress. Acad. Bras. Cienc. 2005;77:695–715. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

