Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools
- PMID: 37569252
- PMCID: PMC10418343
- DOI: 10.3390/ijms241511877
Elucidating the Racemization Mechanism of Aliphatic and Aromatic Amino Acids by In Silico Tools
Abstract
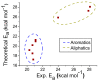
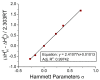
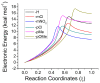
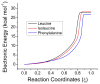
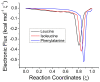
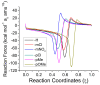
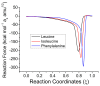
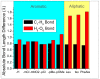
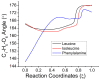
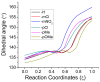
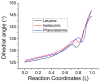
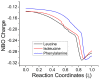
The racemization of biomolecules in the active site can reduce the biological activity of drugs, and the mechanism involved in this process is still not fully comprehended. The present study investigates the impact of aromaticity on racemization using advanced theoretical techniques based on density functional theory. Calculations were performed at the ωb97xd/6-311++g(d,p) level of theory. A compelling explanation for the observed aromatic stabilization via resonance is put forward, involving a carbanion intermediate. The analysis, employing Hammett's parameters, convincingly supports the presence of a negative charge within the transition state of aromatic compounds. Moreover, the combined utilization of natural bond orbital (NBO) analysis and intrinsic reaction coordinate (IRC) calculations confirms the pronounced stabilization of electron distribution within the carbanion intermediate. To enhance our understanding of the racemization process, a thorough examination of the evolution of NBO charges and Wiberg bond indices (WBIs) at all points along the IRC profile is performed. This approach offers valuable insights into the synchronicity parameters governing the racemization reactions.
Keywords: amino acids; density functional theory; intrinsic reaction coordinates; natural bond orbital; racemization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



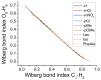
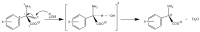
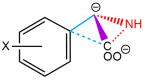
References
-
- Chen Z., Leinisch F., Greco I., Zhang W., Shu N., Chuang C.Y., Lund M.N., Davies M.J. Characterisation and quantification of protein oxidative modifications and amino acid racemisation in powdered infant milk formula. Free Radic. Res. 2019;53:68–81. doi: 10.1080/10715762.2018.1554250. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

