Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts
- PMID: 37569306
- PMCID: PMC10419066
- DOI: 10.3390/ijms241511931
Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts
Abstract
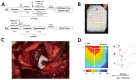
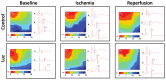
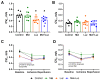
Melatonin has been reported to cause myocardial electrophysiological changes and prevent ventricular tachycardia or fibrillation (VT/VF) in ischemia and reperfusion. We sought to identify electrophysiological targets responsible for the melatonin antiarrhythmic action and to explore whether melatonin receptor-dependent pathways or its antioxidative properties are essential for these effects. Ischemia was induced in anesthetized rats given a placebo, melatonin, and/or luzindole (MT1/MT2 melatonin receptor blocker), and epicardial mapping with reperfusion VT/VFs assessment was performed. The oxidative stress assessment and Western blotting analysis were performed in the explanted hearts. Transmembrane potentials and ionic currents were recorded in cardiomyocytes with melatonin and/or luzindole application. Melatonin reduced reperfusion VT/VF incidence associated with local activation time in logistic regression analysis. Melatonin prevented ischemia-related conduction slowing and did not change the total connexin43 (Cx43) level or oxidative stress markers, but it increased the content of a phosphorylated Cx43 variant (P-Cx43368). Luzindole abolished the melatonin antiarrhythmic effect, slowed conduction, decreased total Cx43, protein kinase Cε and P-Cx43368 levels, and the IK1 current, and caused resting membrane potential (RMP) depolarization. Neither melatonin nor luzindole modified INa current. Thus, the antiarrhythmic effect of melatonin was mediated by the receptor-dependent enhancement of impulse conduction, which was associated with Cx43 phosphorylation and maintaining the RMP level.
Keywords: conduction velocity; connexin-43; melatonin; post-ischemic arrhythmias; potassium current; rat heart; sodium current.
Conflict of interest statement
All authors have indicated they have no potential conflicts of interest to disclose.
Figures
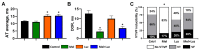


Similar articles
-
Association Between Antiarrhythmic, Electrophysiological, and Antioxidative Effects of Melatonin in Ischemia/Reperfusion.Int J Mol Sci. 2019 Dec 15;20(24):6331. doi: 10.3390/ijms20246331. Int J Mol Sci. 2019. PMID: 31847485 Free PMC article.
-
Melatonin receptor activation protects against low potassium-induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts.J Pineal Res. 2019 Nov;67(4):e12605. doi: 10.1111/jpi.12605. Epub 2019 Sep 10. J Pineal Res. 2019. PMID: 31408542
-
Specific IK1 blockade: a new antiarrhythmic mechanism? Effect of RP58866 on ventricular arrhythmias in rat, rabbit, and primate.Circulation. 1993 Jun;87(6):1979-89. doi: 10.1161/01.cir.87.6.1979. Circulation. 1993. PMID: 8504513
-
Connexin43, A Promising Target to Reduce Cardiac Arrhythmia Burden in Pulmonary Arterial Hypertension.Int J Mol Sci. 2024 Mar 14;25(6):3275. doi: 10.3390/ijms25063275. Int J Mol Sci. 2024. PMID: 38542257 Free PMC article. Review.
-
Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits.Oxid Med Cell Longev. 2021 Mar 13;2021:8876792. doi: 10.1155/2021/8876792. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33791076 Free PMC article. Review.
Cited by
-
The interactions between melatonin and the renin-angiotensin system (RAS) in vascular attenuation in diabetic and non-diabetic conditions.Acta Diabetol. 2025 Jun;62(6):801-809. doi: 10.1007/s00592-025-02479-2. Epub 2025 Mar 13. Acta Diabetol. 2025. PMID: 40080199 Review.
-
Association Between Melatonin Receptor Agonists and Cardiac Arrhythmia; Disproportionality Analysis Studies Using Pharmacovigilance Databases.Cardiovasc Toxicol. 2025 Aug;25(8):1191-1201. doi: 10.1007/s12012-025-10029-z. Epub 2025 Jun 24. Cardiovasc Toxicol. 2025. PMID: 40550964
-
Melatonin as an adjunctive therapy in cardiovascular disease management.Sci Prog. 2024 Oct-Dec;107(4):368504241299993. doi: 10.1177/00368504241299993. Sci Prog. 2024. PMID: 39574322 Free PMC article. Review.
-
Mechanistic Insights into Melatonin's Antiarrhythmic Effects in Acute Ischemia-Reperfusion-Injured Rabbit Hearts Undergoing Therapeutic Hypothermia.Int J Mol Sci. 2025 Jan 13;26(2):615. doi: 10.3390/ijms26020615. Int J Mol Sci. 2025. PMID: 39859328 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

