Nutritional Interventions with Bacillus coagulans Improved Glucose Metabolism and Hyperinsulinemia in Mice with Acute Intermittent Porphyria
- PMID: 37569315
- PMCID: PMC10418637
- DOI: 10.3390/ijms241511938
Nutritional Interventions with Bacillus coagulans Improved Glucose Metabolism and Hyperinsulinemia in Mice with Acute Intermittent Porphyria
Abstract
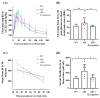
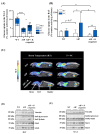
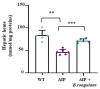
Acute intermittent porphyria (AIP) is a metabolic disorder caused by mutations in the porphobilinogen deaminase (PBGD) gene, encoding the third enzyme of the heme synthesis pathway. Although AIP is characterized by low clinical penetrance (~1% of PBGD mutation carriers), patients with clinically stable disease report chronic symptoms and frequently show insulin resistance. This study aimed to evaluate the beneficial impact of nutritional interventions on correct carbohydrate dysfunctions in a mouse model of AIP that reproduces insulin resistance and altered glucose metabolism. The addition of spores of Bacillus coagulans in drinking water for 12 weeks modified the gut microbiome composition in AIP mice, ameliorated glucose tolerance and hyperinsulinemia, and stimulated fat disposal in adipose tissue. Lipid breakdown may be mediated by muscles burning energy and heat dissipation by brown adipose tissue, resulting in a loss of fatty tissue and improved lean/fat tissue ratio. Probiotic supplementation also improved muscle glucose uptake, as measured using Positron Emission Tomography (PET) analysis. In conclusion, these data provide a proof of concept that probiotics, as a dietary intervention in AIP, induce relevant changes in intestinal bacteria composition and improve glucose uptake and muscular energy utilization. Probiotics may offer a safe, efficient, and cost-effective option to manage people with insulin resistance associated with AIP.
Keywords: glucose homeostasis; gut microbiome; hepatic porphyria; insulin resistance; metabolic disease; nutritional intervention; probiotics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria.Int J Mol Sci. 2022 Dec 20;24(1):51. doi: 10.3390/ijms24010051. Int J Mol Sci. 2022. PMID: 36613492 Free PMC article. Review.
-
Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency.Hum Mol Genet. 2016 Apr 1;25(7):1318-27. doi: 10.1093/hmg/ddw013. Epub 2016 Jan 21. Hum Mol Genet. 2016. PMID: 26908609
-
Systemic messenger RNA replacement therapy is effective in a novel clinically relevant model of acute intermittent porphyria developed in non-human primates.Gut. 2025 Jan 17;74(2):270-283. doi: 10.1136/gutjnl-2024-332619. Gut. 2025. PMID: 39366725 Free PMC article.
-
Porphobilinogen deaminase gene mutations in Polish patients with non-erythroid acute intermittent porphyria.Adv Clin Exp Med. 2015 Jan-Feb;24(1):63-8. doi: 10.17219/acem/34555. Adv Clin Exp Med. 2015. PMID: 25923088
-
May 2006 update in porphobilinogen deaminase gene polymorphisms and mutations causing acute intermittent porphyria: comparison with the situation in Slavic population.Physiol Res. 2006;55 Suppl 2:S119-136. doi: 10.33549/physiolres.930000.55.S2.119. Physiol Res. 2006. PMID: 17298216 Review.
References
-
- Chen B., Solis-Villa C., Hakenberg J., Qiao W., Srinivasan R.R., Yasuda M., Balwani M., Doheny D., Peter I., Chen R., et al. Acute Intermittent Porphyria: Predicted Pathogenicity of HMBS Variants Indicates Extremely Low Penetrance of the Autosomal Dominant Disease. Hum. Mutat. 2016;37:1215–1222. doi: 10.1002/humu.23067. - DOI - PMC - PubMed
-
- Nordmann Y., Puy H., Da Silva V., Simonin S., Robreau A.M., Bonaiti C., Phung L.N., Deybach J.C. Acute Intermittent Porphyria: Prevalence of Mutations in the Porphobilinogen Deaminase Gene in Blood Donors in France. J. Intern. Med. 1997;242:213–217. doi: 10.1046/j.1365-2796.1997.00189.x. - DOI - PubMed
-
- Barreda-Sánchez M., Buendía-Martínez J., Glover-López G., Carazo-Díaz C., Ballesta-Martínez M.J., López-González V., Sánchez-Soler M.J., Rodriguez-Peña L., Serrano-Antón A.T., Gil-Ferrer R., et al. High Penetrance of Acute Intermittent Porphyria in a Spanish Founder Mutation Population and CYP2D6 Genotype as a Susceptibility Factor. Orphanet J. Rare Dis. 2019;14:59. doi: 10.1186/s13023-019-1031-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

