Standardization of a Sex-Sorting Protocol for Stallion Spermatozoa by Means of Absolute RT-qPCR
- PMID: 37569324
- PMCID: PMC10419253
- DOI: 10.3390/ijms241511947
Standardization of a Sex-Sorting Protocol for Stallion Spermatozoa by Means of Absolute RT-qPCR
Abstract
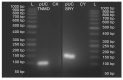
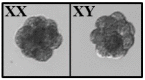
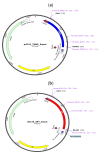
Sperm sexing is a technology that can generate great economic benefits in the animal production sector. Techniques such as sex-sorting promise over 90% accuracy in sperm sexing. However, for the correct standardization of the technique, some laboratory methodologies are required. The present manuscript describes in detail a standardized equine sperm sex-sorting protocol using an absolute qPCR-based methodology. Furthermore, the results of absolute qPCR were implemented and validated by generating equine/bovine heterologous embryos by intracytoplasmic sperm injection (ICSI) of presumably sexed equine spermatozoa into bovine oocytes using a piezoelectric system (Piezo-ICSI). Our results indicated that equine sex-sorting spermatozoa had a 97% and 94% certainty for X and Y sperm, respectively, while presumptive female and male equine/bovine hybrid embryos, generated by Piezo-ICSI, had an accuracy of 92% with respect to the desired sex. Therefore, it is concluded that the presented methodology is a reliable, cost-effective, and relatively simple option for standardizing sex-sorting of equine spermatozoa. This is supported by the results of the correct sexing of Piezo-ICSI heterologous embryos generated with the sexed spermatozoa, validating the correct sexing and viability of these gametes.
Keywords: PIEZO-ICSI; absolute RT-qPCR; plasmids; sex-sorting; sperm stallion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

