Protective Effects of 3'-Epilutein and 3'-Oxolutein against Glutamate-Induced Neuronal Damage
- PMID: 37569384
- PMCID: PMC10418699
- DOI: 10.3390/ijms241512008
Protective Effects of 3'-Epilutein and 3'-Oxolutein against Glutamate-Induced Neuronal Damage
Abstract
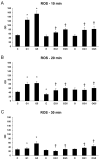
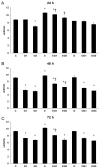
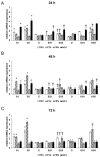
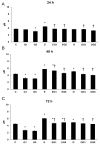
Dietary lutein can be naturally metabolized to 3'-epilutein and 3'-oxolutein in the human body. The epimerization of lutein can happen in acidic pH, and through cooking, 3'-epilutein can be the product of the direct oxidation of lutein in the retina, which is also present in human serum. The 3'-oxolutein is the main oxidation product of lutein. Thus, the allylic oxidation of dietary lutein can result in the formation of 3'-oxolutein, which may undergo reduction either to revert to dietary lutein or epimerize to form 3'-epilutein. We focused on the effects of 3'-epilutein and 3'-oxolutein itself and on glutamate-induced neurotoxicity on SH-SY5Y human neuroblastoma cells to identify the possible alterations in oxidative stress, inflammation, antioxidant capacity, and iron metabolism that affect neurological function. ROS measurements were performed in the differently treated cells. The inflammatory state of cells was followed by TNFα, IL-6, and IL-8 cytokine ELISA measurements. The antioxidant status of the cells was determined by the total antioxidant capacity kit assay. The alterations of genes related to ferroptosis and lipid peroxidation were followed by gene expression measurements; then, thiol measurements were performed. Lutein metabolites 3'-epilutein and 3'-oxolutein differently modulated the effect of glutamate on ROS, inflammation, ferroptosis-related iron metabolism, and lipid peroxidation in SH-SY5Y cells. Our results revealed the antioxidant and anti-inflammatory features of 3'-epilutein and 3'-oxolutein as possible protective agents against glutamate-induced oxidative stress in SH-SY5Y cells, with greater efficacy in the case of 3'-epilutein.
Keywords: 3′-epilutein; 3′-oxolutein; glutamate; neuron; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity.Antioxidants (Basel). 2022 Nov 17;11(11):2269. doi: 10.3390/antiox11112269. Antioxidants (Basel). 2022. PMID: 36421455 Free PMC article.
-
3'-Oxolutein, a metabolite of lutein in chickens.Poult Sci. 1986 Nov;65(11):2135-41. doi: 10.3382/ps.0652135. Poult Sci. 1986. PMID: 3822993
-
Decursin ameliorates neurotoxicity induced by glutamate through restraining ferroptosis by up-regulating FTH1 in SH-SY5Y neuroblastoma cells.Neuroscience. 2024 Nov 1;559:139-149. doi: 10.1016/j.neuroscience.2024.08.035. Epub 2024 Aug 26. Neuroscience. 2024. PMID: 39197742
-
Identification of lutein and zeaxanthin oxidation products in human and monkey retinas.Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1802-11. Invest Ophthalmol Vis Sci. 1997. PMID: 9286269
-
Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators.Int J Mol Sci. 2019 Oct 8;20(19):4968. doi: 10.3390/ijms20194968. Int J Mol Sci. 2019. PMID: 31597407 Free PMC article. Review.
Cited by
-
Bee Pollen Potential to Modulate Ferroptosis: Phytochemical Insights for Age-Related Diseases.Antioxidants (Basel). 2025 Feb 25;14(3):265. doi: 10.3390/antiox14030265. Antioxidants (Basel). 2025. PMID: 40227202 Free PMC article. Review.
-
Lutein Production and Extraction from Microalgae: Recent Insights and Bioactive Potential.Int J Mol Sci. 2024 Mar 1;25(5):2892. doi: 10.3390/ijms25052892. Int J Mol Sci. 2024. PMID: 38474137 Free PMC article. Review.
-
Antioxidant and Antidiabetic Properties of a Thinned-Nectarine-Based Nanoformulation in a Pancreatic β-Cell Line.Antioxidants (Basel). 2023 Dec 31;13(1):63. doi: 10.3390/antiox13010063. Antioxidants (Basel). 2023. PMID: 38247487 Free PMC article.
-
Bee Pollen as a Source of Biopharmaceuticals for Neurodegeneration and Cancer Research: A Scoping Review and Translational Prospects.Molecules. 2024 Dec 13;29(24):5893. doi: 10.3390/molecules29245893. Molecules. 2024. PMID: 39769981 Free PMC article.
-
Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells.Int J Mol Sci. 2024 Sep 5;25(17):9624. doi: 10.3390/ijms25179624. Int J Mol Sci. 2024. PMID: 39273575 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

