Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand?
- PMID: 37569532
- PMCID: PMC10418822
- DOI: 10.3390/ijms241512159
Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand?
Abstract
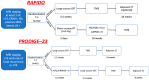
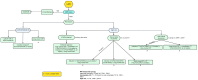
The therapeutic landscape in locally advanced rectal cancer (LARC) has undergone a significant paradigm shift in recent years with the rising adoption of total neoadjuvant treatment (TNT). This comprehensive approach entails administering chemotherapy and radiation therapy before surgery, followed by optional adjuvant chemotherapy. To establish and deliver the optimal tailored treatment regimen to the patient, it is crucial to foster collaboration among a multidisciplinary team comprising healthcare professionals from various specialties, including medical oncology, radiation oncology, surgical oncology, radiology, and pathology. This review aims to provide insights into the current state of TNT for LARC and new emerging strategies to identify potential directions for future research and clinical practice, such as circulating tumor-DNA, immunotherapy in mismatch-repair-deficient tumors, and nonoperative management.
Keywords: MMR-deficient tumor; ct-DNA; locally advanced rectal cancer; nonoperative management; rectal cancer; total neoadjuvant therapy.
Conflict of interest statement
A.S. reports honoraria and advisory board consultancy from BMS (Bristol Myers Squibb), Servier, Gilead, Pfizer, Eisai, Bayer, and MSD (Merck Sharp and Dohme); speaker honoraria from Takeda, BMS, Roche, Abb-Vie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, Lilly, Sandoz, Eisai, Novartis, Bayer, and MSD (all outside the submitted work). The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflict of interest.
Figures


References
-
- Kapiteijn E., Marijnen C.A., Nagtegaal I.D., Putter H., Steup W.H., Wiggers T., Rutten H.J., Pahlman L., Glimelius B., Van Krieken J.H., et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

