ω-3 Polyunsaturated Fatty Acids Improve the Blood-Brain-Barrier Integrity in Contrast-Induced Blood-Brain-Barrier Injury in Uremic Mice
- PMID: 37569545
- PMCID: PMC10418677
- DOI: 10.3390/ijms241512168
ω-3 Polyunsaturated Fatty Acids Improve the Blood-Brain-Barrier Integrity in Contrast-Induced Blood-Brain-Barrier Injury in Uremic Mice
Abstract
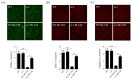
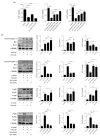
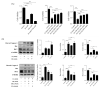
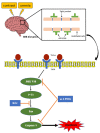
In patients with chronic kidney disease, the need for examinations using contrast media (CM) increases because of underlying diseases. Although contrast agents can affect brain cells, the blood-brain barrier (BBB) protects against brain-cell damage in vivo. However, uremia can disrupt the BBB, increasing the possibility of contrast-agent-induced brain-cell damage in patients with chronic kidney disease (CKD). ω-3 polyunsaturated fatty acids (PUFAs) have shown protective effects on various neurological disorders, including uremic brain injury. This study examined whether ω-3 PUFAs attenuate damage to the BBB caused by uremia and contrast agents in a uremic mouse model and evaluated its associated mechanisms. C57BL/6 mice (eight weeks old, male) and fat-1 mice (b6 background/eight weeks old, male) were divided into groups according to uremic induction, CM, and ω-3 PUFA administration. Uremia was induced via 24 h ischemia-reperfusion (IR) renal injury. One day after CM treatment, the brain tissue, kidney tissue, and blood were collected. The expression levels of glial fibrillary acidic protein (GFAP), claudin 5, CD31, laminin α4, and laminin α5 increased in ω-3 PUFA + CM-treated uremic mice and the brain of fat-1 + CM-treated uremic mice compared with those in the brains of CM-treated uremic mice. The pro-apoptotic protein expression decreased, whereas the anti-apoptotic proteins increased in ω-3 PUFA + CM-treated uremic mice and fat-1 + CM-treated uremic mice compared with CM-treated uremic mice. In addition, the brain-expression levels of p-JNK, p-P53, and p-P38 decreased in the ω-3 PUFA + CM-treated uremic mice and fat-1 + CM-treated uremic mice compared with those in wild-type uremic mice. Our results confirm that uremic toxin and CM damage the BBB and cause brain-cell death. ω-3 PUFAs play a role in BBB protection caused by CM in uremic mice.
Keywords: blood–brain barrier (BBB); contrast media (CM); ischemia–reperfusion; uremic toxin; ω-3 PUFA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Modi K., Padala S.A., Gupta M. Contrast-Induced Nephropathy. StatPearls Publishing; Treasure Island, FL, USA: 2023. - PubMed
-
- Stacul F., on behalf of the Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) van der Molen A.J., Reimer P., Webb J.A.W., Thomsen H.S., Morcos S.K., Almén T., Aspelin P., Bellin M.-F., et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur. Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. - DOI - PubMed
-
- Bobot M., Thomas L., Moyon A., Fernandez S., McKay N., Balasse L., Garrigue P., Brige P., Chopinet S., Poitevin S., et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020;31:1509–1521. doi: 10.1681/ASN.2019070728. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

