Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions
- PMID: 37569642
- PMCID: PMC10418831
- DOI: 10.3390/ijms241512270
Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions
Abstract
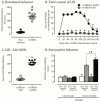
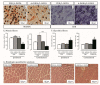
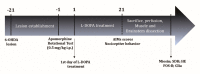
Parkinson's Disease (PD), treated with the dopamine precursor l-3,4-dihydroxyphenylalanine (L-DOPA), displays motor and non-motor orofacial manifestations. We investigated the pathophysiologic mechanisms of the lateral pterygoid muscles (LPMs) and the trigeminal system related to PD-induced orofacial manifestations. A PD rat model was produced by unilateral injection of 6-hydroxydopamine into the medial forebrain bundle. Abnormal involuntary movements (dyskinesia) and nociceptive responses were determined. We analyzed the immunodetection of Fos-B and microglia/astrocytes in trigeminal and facial nuclei and morphological markers in the LPMs. Hyperalgesia response was increased in hemiparkinsonian and dyskinetic rats. Hemiparkinsonism increased slow skeletal myosin fibers in the LPMs, while in the dyskinetic ones, these fibers decreased in the contralateral side of the lesion. Bilateral increased glycolytic metabolism and an inflammatory muscle profile were detected in dyskinetic rats. There was increased Fos-B expression in the spinal nucleus of lesioned rats and in the motor and facial nucleus in L-DOPA-induced dyskinetic rats in the contralateral side of the lesion. Glial cells were increased in the facial nucleus on the contralateral side of the lesion. Overall, spinal trigeminal nucleus activation may be associated with orofacial sensorial impairment in Parkinsonian rats, while a fatigue profile on LPMs is suggested in L-DOPA-induced dyskinesia when the motor and facial nucleus are activated.
Keywords: brain function; muscle biology; neuroscience/neurobiology; neurotoxicity; pain.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Uchida T., Adachi K., Fujita S., Lee J., Gionhaku N., Cools A.R., Koshikawa N. Role of GABA(A) receptors in the retrorubral field and ventral pallidum in rat jaw movementselicited by dopaminergic stimulation of the nucleus accumbens shell. Eur. J. Pharmacol. 2005;510:39–47. doi: 10.1016/j.ejphar.2005.01.012. - DOI - PubMed
-
- Koshikawa N., Fujita S., Adachi K. Behavioral pharmacology of orofacial movement disorders. Int. Rev. Neurobiol. 2011;97:1–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

