Polynucleotides Suppress Inflammation and Stimulate Matrix Synthesis in an In Vitro Cell-Based Osteoarthritis Model
- PMID: 37569659
- PMCID: PMC10418450
- DOI: 10.3390/ijms241512282
Polynucleotides Suppress Inflammation and Stimulate Matrix Synthesis in an In Vitro Cell-Based Osteoarthritis Model
Abstract
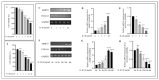
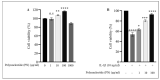
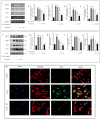
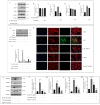
Osteoarthritis (OA) is characterized by degeneration of the joint cartilage, inflammation, and a change in the chondrocyte phenotype. Inflammation also promotes cell hypertrophy in human articular chondrocytes (HC-a) by activating the NF-κB pathway. Chondrocyte hypertrophy and inflammation promote extracellular matrix degradation (ECM). Chondrocytes depend on Smad signaling to control and regulate cell hypertrophy as well as to maintain the ECM. The involvement of these two pathways is crucial for preserving the homeostasis of articular cartilage. In recent years, Polynucleotides Highly Purified Technology (PN-HPT) has emerged as a promising area of research for the treatment of OA. PN-HPT involves the use of polynucleotide-based agents with controlled natural origins and high purification levels. In this study, we focused on evaluating the efficacy of a specific polynucleotide sodium agent, known as CONJURAN, which is derived from fish sperm. Polynucleotides (PN), which are physiologically present in the matrix and function as water-soluble nucleic acids with a gel-like property, have been used to treat patients with OA. However, the specific mechanisms underlying the effect remain unclear. Therefore, we investigated the effect of PN in an OA cell model in which HC-a cells were stimulated with interleukin-1β (IL-1β) with or without PN treatment. The CCK-8 assay was used to assess the cytotoxic effects of PN. Furthermore, the enzyme-linked immunosorbent assay was utilized to detect MMP13 levels, and the nitric oxide assay was utilized to determine the effect of PN on inflammation. The anti-inflammatory effects of PN and related mechanisms were investigated using quantitative PCR, Western blot analysis, and immunofluorescence to examine and analyze relative markers. PN inhibited IL-1β induced destruction of genes and proteins by downregulating the expression of MMP3, MMP13, iNOS, and COX-2 while increasing the expression of aggrecan (ACAN) and collagen II (COL2A1). This study demonstrates, for the first time, that PN exerted anti-inflammatory effects by partially inhibiting the NF-κB pathway and increasing the Smad2/3 pathway. Based on our findings, PN can potentially serve as a treatment for OA.
Keywords: chondrocytes; extracellular matrix; hypertrophy; inflammation; osteoarthritis; polynucleotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Terkawi M.A., Ebata T., Yokota S., Takahashi D., Endo T., Matsumae G., Shimizu T., Kadoya K., Iwasaki N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines. 2022;10:1109. doi: 10.3390/biomedicines10051109. - DOI - PMC - PubMed
-
- Cieza A., Causey K., Kamenov K., Hanson S.W., Chatterji S., Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:2006–2017. doi: 10.1016/S0140-6736(20)32340-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

