Systematic Development of Vanadium Catalysts for Sustainable Epoxidation of Small Alkenes and Allylic Alcohols
- PMID: 37569673
- PMCID: PMC10418365
- DOI: 10.3390/ijms241512299
Systematic Development of Vanadium Catalysts for Sustainable Epoxidation of Small Alkenes and Allylic Alcohols
Abstract
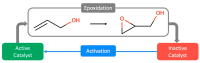
The catalytic epoxidation of small alkenes and allylic alcohols includes a wide range of valuable chemical applications, with many works describing vanadium complexes as suitable catalysts towards sustainable process chemistry. But, given the complexity of these mechanisms, it is not always easy to sort out efficient examples for streamlining sustainable processes and tuning product optimization. In this review, we provide an update on major works of tunable vanadium-catalyzed epoxidations, with a focus on sustainable optimization routes. After presenting the current mechanistic view on vanadium catalysts for small alkenes and allylic alcohols' epoxidation, we argue the key challenges in green process development by highlighting the value of updated kinetic and mechanistic studies, along with essential computational studies.
Keywords: alkene epoxidation; allylic alcohol epoxidation; sustainable chemistry; vanadium catalysts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Vanadium-catalyzed asymmetric epoxidation of allylic alcohols in water.J Org Chem. 2009 May 1;74(9):3350-5. doi: 10.1021/jo900294h. J Org Chem. 2009. PMID: 19344136
-
Asymmetric epoxidation of allylic alcohols catalyzed by vanadium-binaphthylbishydroxamic Acid complex.J Org Chem. 2015 Mar 20;80(6):3203-10. doi: 10.1021/acs.joc.5b00185. Epub 2015 Mar 6. J Org Chem. 2015. PMID: 25714329
-
Enantioselective epoxidation of allylic alcohols by a chiral complex of vanadium: an effective controller system and a rational mechanistic model.Angew Chem Int Ed Engl. 2005 Jul 11;44(28):4389-91. doi: 10.1002/anie.200500938. Angew Chem Int Ed Engl. 2005. PMID: 15942964 No abstract available.
-
Tandem reactions for streamlining synthesis: enantio- and diastereoselective one-pot generation of functionalized epoxy alcohols.Acc Chem Res. 2008 Aug;41(8):883-93. doi: 10.1021/ar800006h. Acc Chem Res. 2008. PMID: 18710197 Free PMC article. Review.
-
Supported metalloporphyrin catalysts for alkene epoxidation.Org Biomol Chem. 2006 Feb 21;4(4):599-609. doi: 10.1039/b509985d. Epub 2005 Dec 20. Org Biomol Chem. 2006. PMID: 16467931 Review.
Cited by
-
Graphene oxide supported oxidovanadium coordination compound as an efficient catalyst for the green oxidation of benzyl alcohol.Sci Rep. 2025 Jul 2;15(1):23027. doi: 10.1038/s41598-025-07345-3. Sci Rep. 2025. PMID: 40596244 Free PMC article.
-
Coordination Polymers of Vanadium and Selected Metal Ions with N,O-Donor Schiff Base Ligands-Synthesis, Crystal Structure, and Application.Molecules. 2025 Feb 27;30(5):1104. doi: 10.3390/molecules30051104. Molecules. 2025. PMID: 40076327 Free PMC article. Review.
References
-
- Nielsen L.P.C., Jacobsen E.N. Aziridines and Epoxides in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2006. Catalytic Asymmetric Epoxide Ring-opening Chemistry; pp. 229–269.
-
- Padwa A., Murphree S. Epoxides and aziridines—A mini review. ARKIVOC. 2005;2006:6–33. doi: 10.3998/ark.5550190.0007.302. - DOI
-
- Molander G.A., Mautner K. Novel processes for stereoselective organic synthesis. Pure Appl. Chem. 1990;62:707–712. doi: 10.1351/pac199062040707. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

