Personalized CFTR Modulator Therapy for G85E and N1303K Homozygous Patients with Cystic Fibrosis
- PMID: 37569738
- PMCID: PMC10418744
- DOI: 10.3390/ijms241512365
Personalized CFTR Modulator Therapy for G85E and N1303K Homozygous Patients with Cystic Fibrosis
Abstract
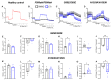
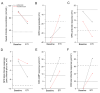
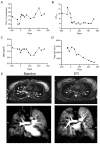
CFTR modulator therapy with elexacaftor/tezacaftor/ivacaftor (ETI) has been approved for people with CF and at least one F508del allele in Europe. In the US, the ETI label has been expanded to 177 rare CFTR mutations responsive in Fischer rat thyroid cells, including G85E, but not N1303K. However, knowledge on the effect of ETI on G85E or N1303K CFTR function remains limited. In vitro effects of ETI were measured in primary human nasal epithelial cultures (pHNECs) of a G85E homozygous patient and an N1303K homozygous patient. Effects of ETI therapy in vivo in these patients were assessed using clinical outcomes, including multiple breath washout and lung MRI, and the CFTR biomarkers sweat chloride concentration (SCC), nasal potential difference (NPD) and intestinal current measurement (ICM), before and after initiation of ETI. ETI increased CFTR-mediated chloride transport in G85E/G85E and N1303K/N1303K pHNECs. In the G85E/G85E and the N1303K/N1303K patient, we observed an improvement in lung function, SCC, and CFTR function in the respiratory and rectal epithelium after initiation of ETI. The approach of combining preclinical in vitro testing with subsequent in vivo verification can facilitate access to CFTR modulator therapy and enhance precision medicine for patients carrying rare CFTR mutations.
Keywords: CFTR; CFTR modulator; G85E; N1303K; cystic fibrosis; human nasal epithelial cells; intestinal current measurement; nasal potential difference.
Conflict of interest statement
S.Y.G. reports grants from Vertex Pharmaceuticals; and lecture honoraria from Chiesi and Vertex Pharmaceuticals; and advisory board participation for Chiesi and Vertex Pharmaceuticals, outside the submitted work. M.AM. reports grants from Vertex Pharmaceuticals; fees for advisory board participation or consulting from Abbvie, Antabio, Arrowhead Pharmaceuticals, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pari, Prieris, Recode, Santhera, Splisense, Vertex Pharmaceuticals; lecture honoraria from Vertex Pharmaceuticals; travel support from Boehringer Ingelheim and Vertex Pharmaceuticals outside the submitted work. J.R. received lecture honoraria from Vertex Pharmaceuticals outside the submitted work. M.S. reports grants from Vertex Pharmaceuticals; and lecture honoraria from Vertex Pharmaceuticals; and advisory board participation for Vertex Pharmaceuticals, outside the submitted work. All other authors declare no conflict of interest.
Figures

References
-
- Barry P.J., Mall M.A., Alvarez A., Colombo C., de Winter-de Groot K.M., Fajac I., McBennett K.A., McKone E.F., Ramsey B.W., Sutharsan S., et al. Triple Therapy for Cystic Fibrosis Phe508del-Gating and -Residual Function Genotypes. N. Engl. J. Med. 2021;385:815–825. doi: 10.1056/NEJMoa2100665. - DOI - PMC - PubMed
-
- Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., Mall M.A., Welter J.J., Ramsey B.W., McKee C.M., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
-
- Mall M.A., Brugha R., Gartner S., Legg J., Moeller A., Mondejar-Lopez P., Prais D., Pressler T., Ratjen F., Reix P., et al. Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 Through 11 Years of Age with Cystic Fibrosis Heterozygous for F508del and a Minimal Function Mutation: A Phase 3b, Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med. 2022;206:1361–1369. doi: 10.1164/rccm.202202-0392OC. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

