Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions
- PMID: 37569742
- PMCID: PMC10418848
- DOI: 10.3390/ijms241512367
Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions
Abstract
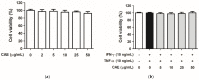
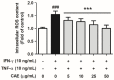
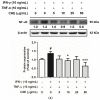
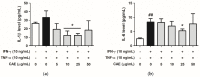
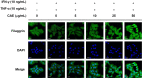
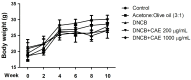
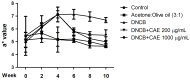
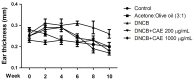
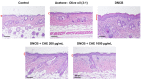
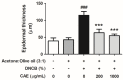
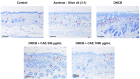
Atopic dermatitis (AD) is a common skin disease worldwide. The major causes of AD are skin barrier defects, immune dysfunction, and oxidative stress. In this study, we investigated the anti-oxidation and anti-inflammation effects of Coffea arabica extract (CAE) and its regulation of the skin barrier and immune functions in AD. In vitro experiments revealed that CAE decreased the reactive oxygen species levels and inhibited the translocation of nuclear factor-κB (NF-κB), further reducing the secretion of interleukin (IL)-1β and IL-6 induced by interferon-γ (IFN-γ)/tumor necrosis factor-α (TNF-α). Moreover, CAE decreased IFN-γ/TNF-α-induced NLR family pyrin domain-containing 3 (NLRP3), caspase-1, high-mobility group box 1 (HMGB1), and receptor for advanced glycation end products (RAGE) expression levels. It also restored the protein levels of skin barrier function-related markers including filaggrin and claudin-1. In vivo experiments revealed that CAE not only reduced the redness of the backs of mice caused by 2,4-dinitrochlorobenzene (DNCB) but also reduced the levels of pro-inflammatory factors in their skin. CAE also reduced transepidermal water loss (TEWL) and immune cell infiltration in DNCB-treated mice. Overall, CAE exerted anti-oxidation and anti-inflammation effects and ameliorated skin barrier dysfunction, suggesting its potential as an active ingredient for AD treatment.
Keywords: Coffea arabica; anti-inflammation; anti-oxidation; atopic dermatitis; immune; inflammasome; skin barrier function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Nguyen M., Pona A., Kolli S.S., Feldman S.R., Strowd L.C. Recent insights in atopic dermatitis pathogenesis, treatment, and disease impact. J. Dermatol. Dermatol. Surg. 2019;23:66–72.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

