Asbestos and Iron
- PMID: 37569765
- PMCID: PMC10419076
- DOI: 10.3390/ijms241512390
Asbestos and Iron
Abstract
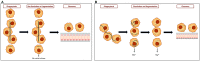
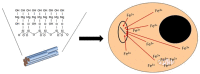
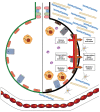
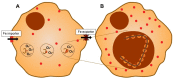
Theories of disease pathogenesis following asbestos exposure have focused on the participation of iron. After exposure, an open network of negatively charged functional groups on the fiber surface complexes host metals with a preference for iron. Competition for iron between the host and the asbestos results in a functional metal deficiency. The homeostasis of iron in the host is modified by the cell response, including increased import to correct the loss of the metal to the fiber surface. The biological effects of asbestos develop in response to and are associated with the disruption of iron homeostasis. Cell iron deficiency in the host following fiber exposure activates kinases and transcription factors, which are associated with the release of mediators coordinating both inflammatory and fibrotic responses. Relative to serpentine chrysotile, the clearance of amphiboles is incomplete, resulting in translocation to the mesothelial surface of the pleura. Since the biological effect of asbestos is dependent on retention of the fiber, the sequestration of iron by the surface, and functional iron deficiency in the cell, the greater clearance (i.e., decreased persistence) of chrysotile results in its diminished impact. An inability to clear asbestos from the lower respiratory tract initiates a host process of iron biomineralization (i.e., asbestos body formation). Host cells attempt to mobilize the metal sequestered by the fiber surface by producing superoxide at the phagosome membrane. The subsequent ferrous cation is oxidized and undergoes hydrolysis, creating poorly crystalline iron oxyhydroxide (i.e., ferrihydrite) included in the coat of the asbestos body.
Keywords: alveolar macrophages; asbestos; ferritin; iron; lung diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

