CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo
- PMID: 37569801
- PMCID: PMC10418880
- DOI: 10.3390/ijms241512427
CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo
Abstract
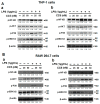
Inflammation is associated with many pathology disorders and the malignant progression of most cancers. Therefore, targeting inflammatory pathways could provide a promising strategy for disease prevention and treatment. In this study, we experimentally investigated the anti-inflammatory effect of CC5 and CC8, two disintegrin isoforms isolated from Cerastes cerastes snake venom, on LPS-stimulated macrophages, both on human THP-1 and mouse RAW264.7 cell adherence and their underlying mechanisms by measuring cytokine release levels and Western blot assay. Equally, both molecules were evaluated on a carrageenan-induced edema rat model. Our findings suggest that CC5 and CC8 were able to reduce adhesion of LPS-stimulated macrophages both on human THP-1 and mouse RAW264.7 cells to fibrinogen and vitronectin through the interaction with the αvβ3 integrin receptor. Moreover, CC5 and CC8 reduced the levels of reactive oxygen species (ROS) mediated by the NF-κB, MAPK and AKT signaling pathways that lead to decreased production of the pro-inflammatory cytokines TNF-α, IL-6 and IL-8 and increased secretion of IL-10 in LPS-stimulated THP-1 and RAW264.7 cells. Interestingly, both molecules potently exhibited an anti-inflammatory effect in vivo by reducing paw swelling in rats. In light of these results, we can propose the CC5 and CC8 disintegrins as interesting tools to design potential candidates against inflammatory-related diseases.
Keywords: cytokines; disintegrin; inflammation; signaling pathways; snake venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

