Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy
- PMID: 37569804
- PMCID: PMC10418878
- DOI: 10.3390/ijms241512433
Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy
Abstract
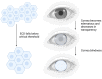
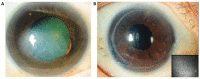
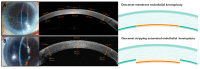
Corneal endothelial dysfunction is one of the leading causes of corneal blindness, and the current conventional treatment option is corneal transplantation using a cadaveric donor cornea. However, there is a global shortage of suitable donor graft material, necessitating the exploration of novel therapeutic approaches. A stem cell-based regenerative medicine approach using induced pluripotent stem cells (iPSCs) offers a promising solution, as they possess self-renewal capabilities, can be derived from adult somatic cells, and can be differentiated into all cell types including corneal endothelial cells (CECs). This review discusses the progress and challenges in developing protocols to induce iPSCs into CECs, focusing on the different media formulations used to differentiate iPSCs to neural crest cells (NCCs) and subsequently to CECs, as well as the characterization methods and markers that define iPSC-derived CECs. The hurdles and solutions for the clinical application of iPSC-derived cell therapy are also addressed, including the establishment of protocols that adhere to good manufacturing practice (GMP) guidelines. The potential risks of genetic mutations in iPSC-derived CECs associated with long-term in vitro culture and the danger of potential tumorigenicity following transplantation are evaluated. In all, this review provides insights into the advancement and obstacles of using iPSC in the treatment of corneal endothelial dysfunction.
Keywords: cell therapy; corneal endothelial; iPSC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

