Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer
- PMID: 37569810
- PMCID: PMC10419855
- DOI: 10.3390/ijms241512436
Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer
Abstract
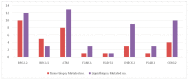
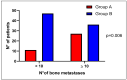
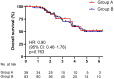
Up to 80% of castration-resistant prostate cancer (CRPC) patients develop bone metastases during the natural history of disease and about 25% harbor mutations in DNA damage repair (DDR) genes. This retrospective observational study evaluated the prevalence of DDR alterations in CRPC patients and their effect on the clinical outcomes associated with bone metastases. The mutational status of CRPC patients was analyzed per FoundationOne® analysis in tissue biopsy or, when it was not possible, in liquid biopsy performed at the onset of metastatic CRPC (mCRPC). The impact of DDR gene mutations on bone-related efficacy endpoints was evaluated at the time of mCRPC diagnoses. In total, 121 mCRPC patients with bone metastases were included: 38 patients had mutations in at least one DDR gene, the remaining 83 ones had a non-mutated DDR status. DDR mutated status was associated with bone metastases volume (p = 0.006), but did not affect SRE (skeletal-related events) incidence and time to SRE onset. Liquid and tissue biopsies were both available for 61 patients with no statistically significant difference in terms of incidence and type of molecular DDR alterations. Mutated DDR status was associated with higher bone metastasic volume, although a not detrimental effect on the other bone-related efficacy endpoints was observed.
Keywords: DDR deficiency; DNA damage repair; SRE; bone metastases; liquid biopsy; mCRPC; prostate cancer; sheletal related events.
Conflict of interest statement
U.D.G: Consultant: Janssen, AstellasPharma, Sanofi, Bayer, Pfizer, Bristol-Myers Squibb, Novartis, Ipsen, Merck; Institutional funding: Roche, Sanofi, AstraZeneca. EFG: travel accommodation from Janssen-Cilag; M.C.C. travel accommodation from IPSEN; V.C. has served as a consultant/advisory board member for Janssen, Astellas, Merck, AstraZeneca, Amgen and Bayer and has received speaker honoraria or travel support from Astellas, Janssen, Ipsen, Bayer, Bristol and Sanofi; D.S. Onoraria per adv board con janssen MSD Merck Daiichi Sankyo Novartis Astellas roche SERVIER EISAI Gilead Incyte; F.P. advisory board per Lilly, Gilead, Novartis, AstraZeneca.
Figures
References
-
- Abida W., Armenia J., Gopalan A., Brennan R., Walsh M., Barron D., Danila D., Rathkopf D., Morris M., Slovin S., et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00029. - DOI - PMC - PubMed
-
- Mateo J., Porta N., Bianchini D., McGovern U., Elliott T., Jones R., Syndikus I., Ralph C., Jain S., Varughese M., et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;21:162–174. doi: 10.1016/S1470-2045(19)30684-9. - DOI - PMC - PubMed
-
- Abida W., Patnaik A., Campbell D., Shapiro J., Bryce A.H., McDermott R., Sautois B., Vogelzang N.J., Bambury R.M., Voog E., et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring aBRCA1orBRCA2gene alteration. J. Clin. Oncol. 2020;38:3763–3772. doi: 10.1200/JCO.20.01035. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

