Honey Bee Larval Hemolymph as a Source of Key Nutrients and Proteins Offers a Promising Medium for Varroa destructor Artificial Rearing
- PMID: 37569818
- PMCID: PMC10419257
- DOI: 10.3390/ijms241512443
Honey Bee Larval Hemolymph as a Source of Key Nutrients and Proteins Offers a Promising Medium for Varroa destructor Artificial Rearing
Abstract
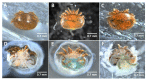
Varroa destructor, a major ectoparasite of the Western honey bee Apis mellifera, is a widespread pest that damages colonies in the Northern Hemisphere. Throughout their lifecycle, V. destructor females feed on almost every developmental stage of their host, from the last larval instar to the adult. The parasite is thought to feed on hemolymph and fat body, although its exact diet and nutritional requirements are poorly known. Using artificial Parafilm™ dummies, we explored the nutrition of V. destructor females and assessed their survival when fed on hemolymph from bee larvae, pupae, or adults. We compared the results with mites fed on synthetic solutions or filtered larval hemolymph. The results showed that the parasites could survive for several days or weeks on different diets. Bee larval hemolymph yielded the highest survival rates, and filtered larval plasma was sufficient to maintain the mites for 14 days or more. This cell-free solution therefore theoretically contains all the necessary nutrients for mite survival. Because some bee proteins are known to be hijacked without being digested by the parasite, we decided to run a proteomic analysis of larval honey bee plasma to highlight the most common proteins in our samples. A list of 54 proteins was compiled, including several energy metabolism proteins such as Vitellogenin, Hexamerin, or Transferrins. These molecules represent key nutrient candidates that could be crucial for V. destructor survival.
Keywords: artificial dummy; hemolymph; insect physiology; mite; nutrition; parasite; protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Chapman N.C., Colin T., Cook J., Da Silva C.R.B., Gloag R., Hogendoorn K., Howard S.R., Remnant E.J., Roberts J.M.K., Tierney S.M., et al. The final frontier: Ecological and evolutionary dynamics of a global parasite invasion. Biol. Lett. 2023;5:20220589. doi: 10.1098/rsbl.2022.0589. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

