Harnessing the Power of Enteric Glial Cells' Plasticity and Multipotency for Advancing Regenerative Medicine
- PMID: 37569849
- PMCID: PMC10419543
- DOI: 10.3390/ijms241512475
Harnessing the Power of Enteric Glial Cells' Plasticity and Multipotency for Advancing Regenerative Medicine
Abstract
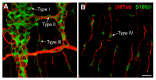
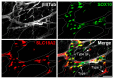
The enteric nervous system (ENS), known as the intrinsic nervous system of the gastrointestinal tract, is composed of a diverse array of neuronal and glial cell subtypes. Fascinating questions surrounding the generation of cellular diversity in the ENS have captivated ENS biologists for a considerable time, particularly with recent advancements in cell type-specific transcriptomics at both population and single-cell levels. However, the current focus of research in this field is predominantly restricted to the study of enteric neuron subtypes, while the investigation of enteric glia subtypes significantly lags behind. Despite this, enteric glial cells (EGCs) are increasingly recognized as equally important regulators of numerous bowel functions. Moreover, a subset of postnatal EGCs exhibits remarkable plasticity and multipotency, distinguishing them as critical entities in the context of advancing regenerative medicine. In this review, we aim to provide an updated overview of the current knowledge on this subject, while also identifying key questions that necessitate future exploration.
Keywords: Hirschsprung disease; Schwann cells; diversity; enteric glial cells; inflammatory bowel diseases; multipotency; neural crest cells; plasticity; regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

