Dihydroxyphenylacetaldehyde Lowering Treatment Improves Locomotor and Neurochemical Abnormalities in the Rat Rotenone Model: Relevance to the Catecholaldehyde Hypothesis for the Pathogenesis of Parkinson's Disease
- PMID: 37569897
- PMCID: PMC10419703
- DOI: 10.3390/ijms241512522
Dihydroxyphenylacetaldehyde Lowering Treatment Improves Locomotor and Neurochemical Abnormalities in the Rat Rotenone Model: Relevance to the Catecholaldehyde Hypothesis for the Pathogenesis of Parkinson's Disease
Abstract
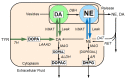
The catecholaldehyde hypothesis for the pathogenesis of Parkinson's disease centers on accumulation of 3,4-dihydroxyphenylacetaldehyde (DOPAL) in dopaminergic neurons. To test the hypothesis, it is necessary to reduce DOPAL and assess if this improves locomotor abnormalities. Systemic administration of rotenone to rats reproduces the motor and central neurochemical abnormalities characterizing Parkinson's disease. In this study, we used the monoamine oxidase inhibitor (MAOI) deprenyl to decrease DOPAL production, with or without the antioxidant N-acetylcysteine (NAC). Adult rats received subcutaneous vehicle, rotenone (2 mg/kg/day via a minipump), or rotenone with deprenyl (5 mg/kg/day i.p.) with or without oral NAC (1 mg/kg/day) for 28 days. Motor function tests included measures of open field activity and rearing. Striatal tissue was assayed for contents of dopamine, DOPAL, and other catechols. Compared to vehicle, rotenone reduced locomotor activity (distance, velocity and rearing); increased tissue DOPAL; and decreased dopamine concentrations and inhibited vesicular sequestration of cytoplasmic dopamine and enzymatic breakdown of cytoplasmic DOPAL by aldehyde dehydrogenase (ALDH), as indicated by DA/DOPAL and DOPAC/DOPAL ratios. The addition of deprenyl to rotenone improved all the locomotor indices, increased dopamine and decreased DOPAL contents, and corrected the rotenone-induced vesicular uptake and ALDH abnormalities. The beneficial effects were augmented when NAC was added to deprenyl. Rotenone evokes locomotor and striatal neurochemical abnormalities found in Parkinson's disease, including DOPAL buildup. Administration of an MAOI attenuates these abnormalities, and NAC augments the beneficial effects. The results indicate a pathogenic role of DOPAL in the rotenone model and suggest that treatment with MAOI+NAC might be beneficial for Parkinson's disease treatment.
Keywords: DOPAL; Parkinson’s disease; catecholaldehydes; monoaminoxidase inhibitor; n-acetylcysteine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

