Molecular Mechanisms of Endothelialitis in SARS-CoV-2 Infection: Evidence for VE-Cadherin Cleavage by ACE2
- PMID: 37569899
- PMCID: PMC10419376
- DOI: 10.3390/ijms241512525
Molecular Mechanisms of Endothelialitis in SARS-CoV-2 Infection: Evidence for VE-Cadherin Cleavage by ACE2
Abstract
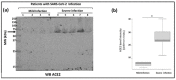
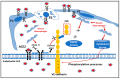
Long COVID-19 syndrome appears after Severe Acute Respiratory Syndrome-Corona Virus (SARS-CoV-2) infection with acute damage to microcapillaries, microthrombi, and endothelialitis. However, the mechanisms involved in these processes remain to be elucidated. All blood vessels are lined with a monolayer of endothelial cells called vascular endothelium, which provides a the major function is to prevent coagulation. A component of endothelial cell junctions is VE-cadherin, which is responsible for maintaining the integrity of the vessels through homophilic interactions of its Ca++-dependent adhesive extracellular domain. Here we provide the first evidence that VE-cadherin is a target in vitro for ACE2 cleavage because its extracellular domain (hrVE-ED) contains two amino acid sequences for ACE2 substrate recognition at the positions 256P-F257 and 321PMKP-325L. Indeed, incubation of hrVE-ED with the active ectopeptidase hrACE2 for 16 hrs in the presence of 10 μM ZnCl2 showed a dose-dependent (from 0.2 ng/μL to 2 ng/μL) decrease of the VE-cadherin immunoreactive band. In vivo, in the blood from patients having severe COVID-19 we detected a circulating form of ACE2 with an apparent molecular mass of 70 kDa, which was barely detectable in patients with mild COVID-19. Of importance, in the patients with severe COVID-19 disease, the presence of three soluble fragments of VE-cadherin (70, 62, 54 kDa) were detected using the antiEC1 antibody while only the 54 kDa fragment was present in patients with mild disease. Altogether, these data clearly support a role for ACE2 to cleave VE-cadherin, which leads to potential biomarkers of SARS-CoV-2 infection related with the vascular disease in "Long COVID-19".
Keywords: ACE2; SARS-CoV-2 infection; VE-cadherin; endothelium; long COVID syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. - DOI - PMC - PubMed
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 1 January 2020)]. Available online: https://covid19.who.int.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

