Catalysis of a Diels-Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework
- PMID: 37570002
- PMCID: PMC10419979
- DOI: 10.3390/ma16155298
Catalysis of a Diels-Alder Reaction between Azachalcones and Cyclopentadiene by a Recyclable Copper(II)-PEIP Metal-Organic Framework
Abstract
Metal-organic frameworks (MOFs) have attracted considerable interest as emerging heterogeneous catalysts for organic transformations of synthetic utility. Herein, a Lewis-acidic MOF, {[Cu3(PEIP)2(5-NH2-mBDC)(DMF)]·7DMF}∞, denoted as Cu(ΙΙ)-PEIP, has been synthesized via a one-pot process and deployed as an efficient heterogeneous catalyst for a Diels-Alder cycloaddition. Specifically, the [4 + 2] cycloaddition of 13 substituted azachalcone dienophiles with cyclopentadiene has been investigated. MOF-catalyzed reaction conditions were optimized, leading to the selection of water as the solvent, in the presence of 10% mol sodium dodecyl sulfate (SDS) to address substrate solubility. The Cu(II)-PEIP catalyst showed excellent activity under these green and mild conditions, exhibiting comparable or, in some cases, superior efficiency to a homogeneous catalyst often employed in Diels-Alder reactions, namely, Cu(OTf)2. The nature of the azachalcone substituent played a significant role in the reactivity of the dienophiles, with electron-withdrawing (EW) substituents enhancing conversion and electron-donating (ED) ones exhibiting the opposite effect. Coordinating substituents appeared to enhance the endo selectivity. Importantly, the Cu(II)-PEIP catalyst can be readily isolated from the reaction mixture and recycled up to four times without any significant reduction in conversion or selectivity.
Keywords: Diels–Alder cycloaddition; MOF; green solvent; heterogeneous catalysis; sustainability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

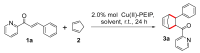


References
-
- Zulkifli Z.I., Lim K.L., Teh L.P. Metal-Organic Frameworks (MOFs) and Their Applications in CO2 Adsorption and Conversion. ChemistrySelect. 2022;7:e202200572. doi: 10.1002/slct.202200572. - DOI
-
- Hu Z., Zhao D. Metal–Organic Frameworks with Lewis Acidity: Synthesis, Characterization, and Catalytic Applications. CrystEngComm. 2017;19:4066–4081. doi: 10.1039/C6CE02660E. - DOI
LinkOut - more resources
Full Text Sources

