Simulation of Corrosion Phenomena in Automotive Components: A Case Study
- PMID: 37570072
- PMCID: PMC10420263
- DOI: 10.3390/ma16155368
Simulation of Corrosion Phenomena in Automotive Components: A Case Study
Abstract
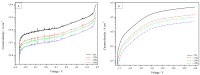
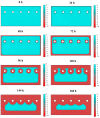
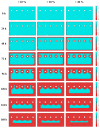
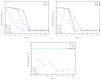
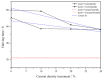
Mathematical modelling and software simulation nowadays are very effective tools for both understanding and predicting corrosion processes and the protection of metallic components. COMSOL Multiphysics 5.6 software provides validated mathematical models that can be used, for a given geometry, as a tool to predict and prevent corrosion of components. The corrosion of zinc-coated steel sheets has been studied in this work by comparing results of the simulations with laboratory tests carried out in a salt spray. Results of both the mathematical modelling and empirical tests give the possibility to estimate the stability of the protective zinc layer over time. The examination of the discrepancies between two analytical methods for the investigation of corrosion phenomena leads to possible modifications in the model in order to reach as much as possible coherence with experimental data. As a final result, a computational model of corrosion phenomena in an automotive component has been reached, allowing in the future to partially substitute laboratory tests, usually being highly time consuming and expensive.
Keywords: coating materials; computer simulations; corrosion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bardal E. Corrosion and Protection. Springer; Berlin/Heidelberg, Germany: 2004.
-
- Wang H., Han E.H. Simulation of Metastable Corrosion Pit Development under Mechanical Stress. Electrochim. Acta. 2013;90:128–134. doi: 10.1016/j.electacta.2012.11.056. - DOI
-
- Mizuno D. Automotive Corrosion and Accelerated Corrosion Tests for Zinc Coated Steels. ISIJ Int. 2018;58:1562–1568. doi: 10.2355/isijinternational.ISIJINT-2018-159. - DOI
-
- Altmayer F. Choosing an Accelerated Corrosion Test. Chicago, Scientific Control Laboratories Inc.; Chicago, IL, USA: 2002.
-
- Meade C.L. Accelerated Corrosion Testing. National Exposure Testing; Sylvania, OH, USA: 2000.
LinkOut - more resources
Full Text Sources
Research Materials

