In Vitro Evaluation of Ag- and Sr-Doped Hydroxyapatite Coatings for Medical Applications
- PMID: 37570133
- PMCID: PMC10419960
- DOI: 10.3390/ma16155428
In Vitro Evaluation of Ag- and Sr-Doped Hydroxyapatite Coatings for Medical Applications
Abstract
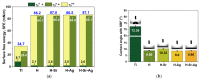
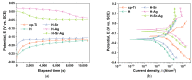
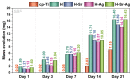
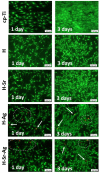
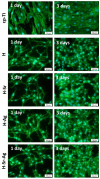
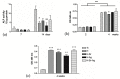
Osseointegration plays the most important role in the success of an implant. One of the applications of hydroxyapatite (HAp) is as a coating for metallic implants due to its bioactive nature, which improves osteoconduction. The purpose of this research was to assess the in vitro behavior of HAp undoped and doped with Ag and/or Sr obtained by galvanostatic pulsed electrochemical deposition. The coatings were investigated in terms of chemical bonds, contact angle and surface free energy, electrochemical behavior, in vitro biomineralization in acellular media (SBF and PBS), and biocompatibility with preosteoblasts cells (MC3T3-E1 cell line). The obtained results highlighted the beneficial impact of Ag and/or Sr on the HAp. The FTIR spectra confirmed the presence of hydroxyapatite within all coatings, while in terms of wettability, the contact angle and surface free energy investigations showed that all surfaces were hydrophilic. The in vitro behavior of MC3T3-E1 indicated that the presence of Sr in the HAp coatings as a unique doping agent or in combination with Ag elicited improved cytocompatibility in terms of cell proliferation and osteogenic differentiation. Therefore, the composite HAp-based coatings showed promising potential for bone regeneration applications.
Keywords: bioactivity; biocompatibility; electrochemical deposition; hydroxyapatite; osteogenic differentiation; silver; strontium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Evaluation of the In Vitro Behavior of Electrochemically Deposited Plate-like Crystal Hydroxyapatite Coatings.Biomimetics (Basel). 2024 Nov 17;9(11):704. doi: 10.3390/biomimetics9110704. Biomimetics (Basel). 2024. PMID: 39590276 Free PMC article.
-
In vitro release of silver ions and expression of osteogenic genes by MC3T3-E1 cell line cultured on nano-hydroxyapatite and silver/strontium-coated titanium plates.Odontology. 2023 Jan;111(1):33-40. doi: 10.1007/s10266-022-00747-z. Epub 2022 Sep 29. Odontology. 2023. PMID: 36173497
-
Electrodeposition of Sr-substituted hydroxyapatite on low modulus beta-type Ti-45Nb and effect on in vitro Sr release and cell response.Mater Sci Eng C Mater Biol Appl. 2020 Mar;108:110425. doi: 10.1016/j.msec.2019.110425. Epub 2019 Nov 14. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31923935
-
Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review.Acta Biomater. 2019 Apr 15;89:14-32. doi: 10.1016/j.actbio.2019.03.006. Epub 2019 Mar 6. Acta Biomater. 2019. PMID: 30851454 Review.
-
Multi-functional bioactive silver- and copper-doped diamond-like carbon coatings for medical implants.Acta Biomater. 2023 Sep 1;167:54-68. doi: 10.1016/j.actbio.2023.06.037. Epub 2023 Jun 29. Acta Biomater. 2023. PMID: 37392935 Review.
Cited by
-
In Vitro Characterization of Hydroxyapatite-Based Coatings Doped with Mg or Zn Electrochemically Deposited on Nanostructured Titanium.Biomimetics (Basel). 2024 Apr 18;9(4):244. doi: 10.3390/biomimetics9040244. Biomimetics (Basel). 2024. PMID: 38667255 Free PMC article.
-
Advanced surface modification techniques for titanium implants: a review of osteogenic and antibacterial strategies.Front Bioeng Biotechnol. 2025 Mar 19;13:1549439. doi: 10.3389/fbioe.2025.1549439. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40177619 Free PMC article. Review.
-
In Vitro Studies on 3D-Printed PLA/HA/GNP Structures for Bone Tissue Regeneration.Biomimetics (Basel). 2024 Jan 19;9(1):55. doi: 10.3390/biomimetics9010055. Biomimetics (Basel). 2024. PMID: 38275452 Free PMC article.
-
Holotomography and atomic force microscopy: a powerful combination to enhance cancer, microbiology and nanotoxicology research.Discov Nano. 2024 Apr 9;19(1):64. doi: 10.1186/s11671-024-04003-x. Discov Nano. 2024. PMID: 38594446 Free PMC article. Review.
-
An Advanced Human Bone Tissue Culture Model for the Assessment of Implant Osteointegration In Vitro.Int J Mol Sci. 2024 May 13;25(10):5322. doi: 10.3390/ijms25105322. Int J Mol Sci. 2024. PMID: 38791362 Free PMC article.
References
-
- Chug A., Shukla S., Mahesh L., Jadwani S. Osseointegration—Molecular Events at the Bone–Implant Interface: A Review. J. Oral Maxillofac. Surg. Med. Pathol. 2013;25:1–4. doi: 10.1016/j.ajoms.2012.01.008. - DOI
-
- Khan S.N., Ramachandran M., Senthil Kumar S., Krishnan V., Sundaram R. Osseointegration and More—A Review of Literature. Indian J. Dent. 2012;3:72–76. doi: 10.1016/j.ijd.2012.03.012. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

