Recent Progress in Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors
- PMID: 37570506
- PMCID: PMC10421214
- DOI: 10.3390/nano13152188
Recent Progress in Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors
Abstract
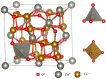
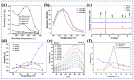
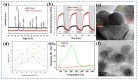
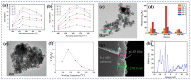
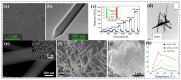
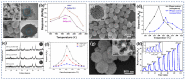
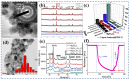
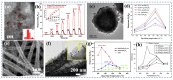
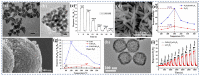
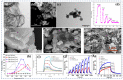
Gas-sensing technology has gained significant attention in recent years due to the increasing concern for environmental safety and human health caused by reactive gases. In particular, spinel ferrite (MFe2O4), a metal oxide semiconductor with a spinel structure, has emerged as a promising material for gas-sensing applications. This review article aims to provide an overview of the latest developments in spinel-ferrite-based gas sensors. It begins by discussing the gas-sensing mechanism of spinel ferrite sensors, which involves the interaction between the target gas molecules and the surface of the sensor material. The unique properties of spinel ferrite, such as its high surface area, tunable bandgap, and excellent stability, contribute to its gas-sensing capabilities. The article then delves into recent advancements in gas sensors based on spinel ferrite, focusing on various aspects such as microstructures, element doping, and heterostructure materials. The microstructure of spinel ferrite can be tailored to enhance the gas-sensing performance by controlling factors such as the grain size, porosity, and surface area. Element doping, such as incorporating transition metal ions, can further enhance the gas-sensing properties by modifying the electronic structure and surface chemistry of the sensor material. Additionally, the integration of spinel ferrite with other semiconductors in heterostructure configurations has shown potential for improving the selectivity and overall sensing performance. Furthermore, the article suggests that the combination of spinel ferrite and semiconductors can enhance the selectivity, stability, and sensing performance of gas sensors at room or low temperatures. This is particularly important for practical applications where real-time and accurate gas detection is crucial. In conclusion, this review highlights the potential of spinel-ferrite-based gas sensors and provides insights into the latest advancements in this field. The combination of spinel ferrite with other materials and the optimization of sensor parameters offer opportunities for the development of highly efficient and reliable gas-sensing devices for early detection and warning systems.
Keywords: chemiresistive gas sensor; doping; heterostructure; metal oxide semiconductor; nanostructure; spinel ferrite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nadargi D.Y., Umar A., Nadargi J.D., Lokare S.A., Akbar S., Mulla I.S., Suryavanshi S.S., Bhandari N.L., Chaskar M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023;58:559–582.
-
- Das S., Jayaraman V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014;66:112–255.
-
- Kurugundla G.K., Godavarti U., Saidireddy P., Pothukanuri N. Zinc oxide based gas sensors and their derivatives: A critical review. J. Mater. Chem. C. 2023;11:3906–3925.
-
- Tian X., Cui X., Lai T., Ren J., Yang Z., Xiao M., Wang B., Xiao X., Wang Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021;3:390–403. doi: 10.1016/j.nanoms.2021.05.011. - DOI
Publication types
Grants and funding
- 62273134, 62173129/National Natural Science Foundation of China
- 21IRTSTHN006/Program for Science & Technology Innovative Research Team in the University of Henan Province
- 212300410042/Natural Science Foundation of Henan Province
- 232102220009/Key Science and Technology Program of Henan Province
- NSFRF220101, NSFRF230432/Fundamental Research Funds for the Universities of Henan Province
LinkOut - more resources
Full Text Sources

