Photoswitchable Molecular Units with Tunable Nonlinear Optical Activity: A Theoretical Investigation
- PMID: 37570617
- PMCID: PMC10419997
- DOI: 10.3390/molecules28155646
Photoswitchable Molecular Units with Tunable Nonlinear Optical Activity: A Theoretical Investigation
Abstract
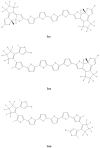
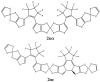
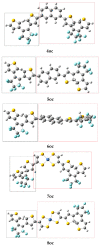
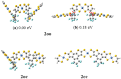
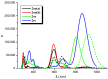
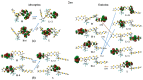
The first-, second-, and third-order molecular nonlinear optical properties, including two-photon absorption of a series of derivatives, involving two dithienylethene (DTE) groups connected by several molecular linkers (bis(ethylene-1,2-dithiolato)Ni- (NiBDT), naphthalene, quasilinear oligothiophene chains), are investigated by employing density functional theory (DFT). These properties can be efficiently controlled by DTE switches, in connection with light of appropriate frequency. NiBDT, as a linker, is associated with a greater contrast, in comparison to naphthalene, between the first and second hyperpolarizabilities of the "open-open" and the "closed-closed" isomers. This is explained by invoking the low-lying excited states of NiBDT. It is shown that the second hyperpolarizability can be used as an index, which follows the structural changes induced by photochromism. Assuming a Förster type transfer mechanism, the intramolecular excited-state energy transfer (EET) mechanism is studied. Two important parameters related to this are computed: the electronic coupling (VDA) between the donor and acceptor fragments as well as the overlap between the absorption and emission spectra of the donor and acceptor groups. NiBDT as a linker is associated with a low electronic coupling, VDA, value. We found that VDA is affected by molecular geometry. Our results predict that the linker strongly influences the communication between the open-closed DTE groups. The sensitivity of the molecular nonlinear optical properties could assist with identification of molecular isomers.
Keywords: (hyper)polarizability; bis(ethylene-1,2-dithiolato); density functional theory; dithienylethene; excited-state energy transfer; molecular switches; photochromism; two-photon absorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Galanti A., Santoro J., Mannancherry R., Duez Q., Diez-Cabanes V., Valasek M., De Winter J., Cornil J., Gerbaux P., Mayor M., et al. A New Class of Rigid Multi(azobenzene) Switches Featuring Electronic Decoupling: Unravelling the Isomerization in Individual Photochromes. J. Am. Chem. Soc. 2019;141:9273–9283. doi: 10.1021/jacs.9b02544. - DOI - PubMed
-
- Zhang L.Y., Friesner R.A., Murphy R.B. Ab initio quantum chemical calculation of electron transfer matrix elements for large molecules. J. Chem. Phys. 1997;107:450–459. doi: 10.1063/1.474406. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

