Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents
- PMID: 37570832
- PMCID: PMC10420935
- DOI: 10.3390/molecules28155860
Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents
Abstract
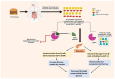
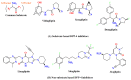
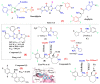
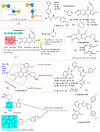
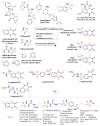
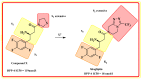
This article sheds light on the various scaffolds that can be used in the designing and development of novel synthetic compounds to create DPP-4 inhibitors for the treatment of type 2 diabetes mellitus (T2DM). This review highlights a variety of scaffolds with high DPP-4 inhibition activity, such as pyrazolopyrimidine, tetrahydro pyridopyrimidine, uracil-based benzoic acid and esters, triazole-based, fluorophenyl-based, glycinamide, glycolamide, β-carbonyl 1,2,4-triazole, and quinazoline motifs. The article further explains that the potential of the compounds can be increased by substituting atoms such as fluorine, chlorine, and bromine. Docking of existing drugs like sitagliptin, saxagliptin, and vildagliptin was done using Maestro 12.5, and the interaction with specific residues was studied to gain a better understanding of the active sites of DPP-4. The structural activities of the various scaffolds against DPP-4 were further illustrated by their inhibitory concentration (IC50) values. Additionally, various synthesis schemes were developed to make several commercially available DPP4 inhibitors such as vildagliptin, sitagliptin and omarigliptin. In conclusion, the use of halogenated scaffolds for the development of DPP-4 inhibitors is likely to be an area of increasing interest in the future.
Keywords: DPP-4 inhibitors; SAR; bio-activity; heterocyclic scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- He L., Wang J., Ping F., Yang N., Huang J., Li W., Xu L., Zhang H., Li Y. Dipeptidyl peptidase-4 inhibitors and gallbladder or biliary disease in type 2 diabetes: Systematic review and pairwise and network meta-analysis of randomised controlled trials. BMJ. 2022;377:E068882. doi: 10.1136/bmj-2021-068882. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

