Sporozoite immunization: innovative translational science to support the fight against malaria
- PMID: 37571809
- PMCID: PMC10949369
- DOI: 10.1080/14760584.2023.2245890
Sporozoite immunization: innovative translational science to support the fight against malaria
Abstract
Introduction: Malaria, a devastating febrile illness caused by protozoan parasites, sickened 247,000,000 people in 2021 and killed 619,000, mostly children and pregnant women in sub-Saharan Africa. A highly effective vaccine is urgently needed, especially for Plasmodium falciparum (Pf), the deadliest human malaria parasite.
Areas covered: Sporozoites (SPZ), the parasite stage transmitted by Anopheles mosquitoes to humans, are the only vaccine immunogen achieving >90% efficacy against Pf infection. This review describes >30 clinical trials of PfSPZ vaccines in the U.S.A., Europe, Africa, and Asia, based on first-hand knowledge of the trials and PubMed searches of 'sporozoites,' 'malaria,' and 'vaccines.'
Expert opinion: First generation (radiation-attenuated) PfSPZ vaccines are safe, well tolerated, 80-100% efficacious against homologous controlled human malaria infection (CHMI) and provide 18-19 months protection without boosting in Africa. Second generation chemo-attenuated PfSPZ are more potent, 100% efficacious against stringent heterologous (variant strain) CHMI, but require a co-administered drug, raising safety concerns. Third generation, late liver stage-arresting, replication competent (LARC), genetically-attenuated PfSPZ are expected to be both safe and highly efficacious. Overall, PfSPZ vaccines meet safety, tolerability, and efficacy requirements for protecting pregnant women and travelers exposed to Pf in Africa, with licensure for these populations possible within 5 years. Protecting children and mass vaccination programs to block transmission and eliminate malaria are long-term objectives.
Introduction Malaria, a devastating febrile illness caused by protozoan parasites, sickened 247,000,000 people in 2021 and killed 619,000, mostly children and pregnant women in sub-Saharan Africa. A highly effective vaccine is urgently needed, especially for Plasmodium falciparum (Pf), the deadliest human malaria parasite. Areas covered Sporozoites (SPZ), the parasite stage transmitted by Anopheles mosquitoes to humans, are the only vaccine immunogen achieving >90% efficacy against Pf infection. This review describes >30 clinical trials of PfSPZ vaccines in the U.S.A., Europe, Africa, and Asia, based on first-hand knowledge of the trials and PubMed searches of ‘sporozoites,’ ‘malaria,’ and ‘vaccines.’ Expert opinion First generation (radiation-attenuated) PfSPZ vaccines are safe, well tolerated, 80–100% efficacious against homologous controlled human malaria infection (CHMI) and provide 18–19 months protection without boosting in Africa. Second generation chemo-attenuated PfSPZ are more potent, 100% efficacious against stringent heterologous (variant strain) CHMI, but require a co-administered drug, raising safety concerns. Third generation, late liver stage-arresting, replication competent (LARC), genetically-attenuated PfSPZ are expected to be both safe and highly efficacious. Overall, PfSPZ vaccines meet safety, tolerability, and efficacy requirements for protecting pregnant women and travelers exposed to Pf in Africa, with licensure for these populations possible within 5 years. Protecting children and mass vaccination programs to block transmission and eliminate malaria are long-term objectives.
Keywords: PfSPZ Vaccine; PfSPZ-CVac; PfSPZ-LARC2 Vaccine; Plasmodium falciparum; Sporozoites; malaria vaccines; review; vaccines.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
Thomas L. Richie, L.W. Preston Church, Tooba Murshedkar, Peter F. Billingsley, Eric R. James, Mei-Chun Chen, Yonas Abebe, Natasha KC, Sumana Chakravarty, David Dolberg, B. Kim Lee Sim, and Stephen L. Hoffman are salaried, full-time employees of Sanaria Inc. B. Kim Lee Sim and Stephen L. Hoffman own stock in Sanaria. Benjamin Mordmüller has a consultancy agreement on vaccine development with Nobelpharma Co., Ltd., Japan. Robert W. Sauerwein has stock ownership in TropIQ Health Sciences, Nijmegen, The Netherlands. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


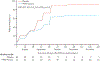
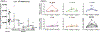


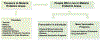
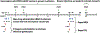

References
-
- Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013. Sep 20;341(6152):p. 1359–1365. - PubMed
-
First demonstration of 100% protection by PfSPZ Vaccine administered by the intravenous route.
-
- Jongo SA, Church LWP, Mtoro AT, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ Vaccine in Tanzanian adults. Clin Infect Dis. 2020. Nov 29;71(11):p. 2849–2857. - PMC - PubMed
-
First demonstration of an inflection point in the dose response curve, whereby higher doses lead to diminished efficacy.
-
- Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. 2022. Nov 18;22(3):p. 377–389. - PMC - PubMed
-
First demonstration that a three dose regimen of PfSPZ Vaccine protects against naturally transmitted Pf malaria in the field.
-
- Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016. May 9;22(6):p. 614–623. - PMC - PubMed
-
First demonstration that PfSPZ Vaccine protects against homologous CHMI for at least 14 months and that intravenous administration is superior to intramuscular administration.
-
- Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A. 2017. Mar 07;114(10):p. 2711–2716. - PMC - PubMed
-
First demonstration of protection against heterologous CHMI by PfSPZ Vaccine that is durable for at least 8 months.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
