Cardiac implantable electronic device implantation and device-related infection
- PMID: 37572046
- PMCID: PMC10422690
- DOI: 10.1093/europace/euad208
Cardiac implantable electronic device implantation and device-related infection
Abstract
Aims: Cardiac implantable electronic devices (CIED) are important tools for managing arrhythmias, improving hemodynamics, and preventing sudden cardiac death. Device-related infections (DRI) remain a significant complication of CIED and are associated with major adverse outcomes. We aimed to assess the trend in CIED implantations, and the burden and morbidity associated with DRI.
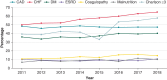
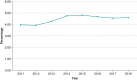
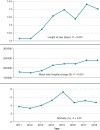
Methods and results: The 2011-2018 National Inpatient Sample database was searched for admissions for CIED implantation and DRI. A total of 1 604 173 admissions for CIED implantations and 71 007 (4.4%) admissions for DRI were reported. There was no significant change in annual admission rates for DRI (3.96-4.59%, P value for trend = 0.98). Those with DRI were more likely to be male (69.3 vs. 57%, P < 0.001) and have a Charlson comorbidity index score ≥3 (46.6 vs. 36.8%, P < 0.001). The prevalence of congestive heart failure (CHF) increased in those admitted with DRI over the observation period. Pulmonary embolism, deep vein thrombosis, and post-procedural hematoma were the most common complications in those with DRI (4.1, 3.6, and 2.90%, respectively). Annual in-hospital mortality for those with DRI ranged from 3.9 to 5.8% (mean 4.4%, P value for trend = 0.07). Multivariate analysis identified CHF [odds ratio (OR) = 1.67; 95% confidence interval (CI) = 1.35-2.07], end-stage renal disease (OR = 1.90; 95% CI = 1.46-2.48), coagulopathy (OR = 2.94; 95% CI = 2.40-3.61), and malnutrition (OR = 2.50; 95% CI = 1.99-3.15) as the predictors of in-hospital mortality for patients admitted with DRI.
Conclusion: Device-related infection is relatively common and continues to be associated with high morbidity and mortality. The prevalence of DRI has not changed significantly despite technical and technological advances in cardiac devices and their implantation.
Keywords: Cardiac implantable electronic device; Cost; Device-related infection; Length of stay; Mortality; Prognosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch Det al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006: implantation trends and patient profiles for pacemakers and ICDs. Pacing Clin Electrophysiol 2010;33:705–11. - PubMed
-
- Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009. J Am Coll Cardiol 2012;60:1540–5. - PubMed
-
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s Project: 2009 survey cardiac pacemakers and ICDS. Pacing Clin Electrophysiol 2011;34:1013–27. - PubMed
-
- Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA 2006;295:809. - PubMed
-
- Boriani G, Ziacchi M, Nesti M, Battista A, Placentino F, Malavasi VLet al. Cardiac resynchronization therapy: how did consensus guidelines from Europe and the United States evolve in the last 15 years? Int J Cardiol 2018;261:119–29. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

