Early brain metabolic disturbances associated with delayed cerebral ischemia in patients with severe subarachnoid hemorrhage
- PMID: 37572080
- PMCID: PMC10676142
- DOI: 10.1177/0271678X231193661
Early brain metabolic disturbances associated with delayed cerebral ischemia in patients with severe subarachnoid hemorrhage
Abstract
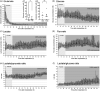
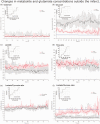
Delayed cerebral ischemia (DCI) is a devastating complication of aneurysmal subarachnoid hemorrhage (ASAH) causing brain infarction and disability. Cerebral microdialysis (CMD) monitoring is a focal technique that may detect DCI-related neurochemical changes as an advance warning. We conducted retrospective analyses from 44 poor-grade ASAH patients and analyzed glucose, lactate, pyruvate, and glutamate concentrations in control patients without DCI (n = 19), and in patients with DCI whose CMD probe was located within (n = 17) or outside (n = 8) a new infarct. When monitored from within a lesion, DCI was preceded by a decrease in glucose and a surge in glutamate, accompanied by increases in lactate/pyruvate and lactate/glucose ratios whereas these parameters remained stable in control patients. When CMD monitoring was performed outside the lesion, the glutamate surge was absent, but glucose and L/G ratio were still significantly altered. Overall, glucose and L/G ratio were significant biomarkers of DCI (se96.0, spe73.7-68.4). Glucose and L/G predicted DCI 67 h before CT detection of a new infarct. The pathogenesis of DCI therefore induces early metabolic disturbances that can be detected by CMD as an advance warning. Glucose and L/G could provide a trigger for initiating further examination or therapy, earlier than when guided by other monitoring techniques.
Keywords: Brain metabolism; cerebral microdialysis; glucose; lactate; multimodal monitoring.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. - PubMed
-
- Charpentier C, Audibert G, Guillemin F, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 1999; 30: 1402–1408. - PubMed
-
- Suarez JI. Diagnosis and management of subarachnoid hemorrhage. Continuum (Minneap Minn) 2015; 21: 1263–1287. - PubMed
-
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–2395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

