Estradiol mitigates stress-induced cardiac injury and inflammation by downregulating ADAM17 via the GPER-1/PI3K signaling pathway
- PMID: 37572114
- PMCID: PMC10423133
- DOI: 10.1007/s00018-023-04886-6
Estradiol mitigates stress-induced cardiac injury and inflammation by downregulating ADAM17 via the GPER-1/PI3K signaling pathway
Abstract
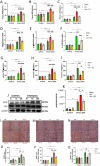
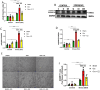
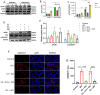
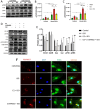
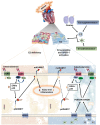
Stress-induced cardiovascular diseases characterized by inflammation are among the leading causes of morbidity and mortality in postmenopausal women worldwide. Estradiol (E2) is known to be cardioprotective via the modulation of inflammatory mediators during stress. But the mechanism is unclear. TNFα, a key player in inflammation, is primarily converted to its active form by 'A Disintegrin and Metalloprotease 17' (ADAM17). We investigated if E2 can regulate ADAM17 during stress. Experiments were performed using female FVB wild-type (WT), C57BL/6 WT, and G protein-coupled estrogen receptor 1 knockout (GPER-1 KO) mice and H9c2 cells. The study revealed a significant increase in cardiac injury and inflammation during isoproterenol (ISO)-induced stress in ovariectomized (OVX) mice. Additionally, ADAM17's membrane content (mADAM17) was remarkably increased in OVX and GPER-1 KO mice during stress. However, in vivo supplementation of E2 significantly reduced cardiac injury, mADAM17, and inflammation. Also, administering G1 (GPER-1 agonist) in mice under stress reduced mADAM17. Further experiments demonstrated that E2, via GPER-1/PI3K pathway, localized ADAM17 at the perinuclear region by normalizing β1AR-Gαs, mediating the switch from β2AR-Gαi to Gαs, and reducing phosphorylated kinases, including p38 MAPKs and ERKs. Thus, using G15 and LY294002 to inhibit GPER-1 and its down signaling molecule, PI3K, respectively, in the presence of E2 during stress resulted in the disappearance of E2's modulatory effect on mADAM17. In vitro knockdown of ADAM17 during stress significantly reduced cardiac injury and inflammation, confirming its significant inflammatory role. These interesting findings provide novel evidence that E2 and G1 are potential therapeutic agents for ADAM17-induced inflammatory diseases associated with postmenopausal females.
Keywords: ADAM17; Cardiac injury; Estradiol; GPER-1; Myocardial inflammation; Stress.
© 2023. The Author(s).
Conflict of interest statement
None declared.
Figures




Similar articles
-
Estradiol contributes to sex differences in resilience to sepsis-induced metabolic dysregulation and dysfunction in the heart via GPER-1-mediated PPARδ/NLRP3 signaling.Metabolism. 2024 Jul;156:155934. doi: 10.1016/j.metabol.2024.155934. Epub 2024 May 16. Metabolism. 2024. PMID: 38762141
-
GPER mediates estrogen cardioprotection against epinephrine-induced stress.J Endocrinol. 2021 May 20;249(3):209-222. doi: 10.1530/JOE-20-0451. J Endocrinol. 2021. PMID: 33847279
-
Mechanistic insights into the anti-fibrotic effects of estrogen via the PI3K-Akt pathway in frozen shoulder.J Steroid Biochem Mol Biol. 2025 May;249:106701. doi: 10.1016/j.jsbmb.2025.106701. Epub 2025 Feb 11. J Steroid Biochem Mol Biol. 2025. PMID: 39947440
-
ADAM17, A Key Player of Cardiac Inflammation and Fibrosis in Heart Failure Development During Chronic Catecholamine Stress.Front Cell Dev Biol. 2021 Dec 13;9:732952. doi: 10.3389/fcell.2021.732952. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34966735 Free PMC article. Review.
-
Protective effects of 17-β-estradiol on liver injury: The role of TLR4 signaling pathway and inflammatory response.Cytokine. 2024 Sep;181:156686. doi: 10.1016/j.cyto.2024.156686. Epub 2024 Jul 10. Cytokine. 2024. PMID: 38991382 Review.
Cited by
-
GPER in metabolic homeostasis and disease: molecular mechanisms, nutritional regulation, and therapeutic potential.J Transl Med. 2025 Aug 26;23(1):960. doi: 10.1186/s12967-025-07005-0. J Transl Med. 2025. PMID: 40859360 Free PMC article. Review.
-
Critical illness and sex hormones: response and impact of the hypothalamic-pituitary-gonadal axis.Ther Adv Endocrinol Metab. 2025 Apr 2;16:20420188251328192. doi: 10.1177/20420188251328192. eCollection 2025. Ther Adv Endocrinol Metab. 2025. PMID: 40183031 Free PMC article. Review.
-
Estrogen via GPER downregulated HIF-1a and MIF expression, attenuated cardiac arrhythmias, and myocardial inflammation during hypobaric hypoxia.Mol Med. 2025 Mar 20;31(1):107. doi: 10.1186/s10020-025-01144-2. Mol Med. 2025. PMID: 40108505 Free PMC article.
-
GPER-1 Rapid Regulation Influences p-Akt Expression to Resist Stress-Induced Injuries in a Sex-Specific Manner.Physiol Res. 2024 Nov 12;73(5):831-839. doi: 10.33549/physiolres.935176. Physiol Res. 2024. PMID: 39530909 Free PMC article.
References
-
- El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142(25):e506–e532. doi: 10.1161/cir.0000000000000912. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

