The nuclear receptor LRH-1 discriminates between ligands using distinct allosteric signaling circuits
- PMID: 37572334
- PMCID: PMC10510465
- DOI: 10.1002/pro.4754
The nuclear receptor LRH-1 discriminates between ligands using distinct allosteric signaling circuits
Abstract
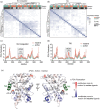
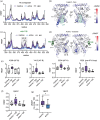
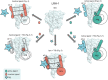
Nuclear receptors (NRs) are transcription factors that regulate essential biological processes in response to cognate ligands. An important part of NR function involves ligand-induced conformational changes that recruit coregulator proteins to the activation function surface (AFS), ~15 Å away from the ligand-binding pocket. Ligands must communicate with the AFS to recruit appropriate coregulators and elicit different transcriptional outcomes, but this communication is poorly understood. These studies illuminate allosteric communication networks underlying activation of liver receptor homolog-1 (LRH-1), a NR that regulates development, metabolism, cancer progression, and intestinal inflammation. Using >100 μs of all-atom molecular dynamics simulations involving 74 LRH-1 complexes, we identify distinct signaling circuits used by active and inactive ligands for AFS communication. Inactive ligands communicate via strong, coordinated motions along paths through the receptor to the AFS. Activating ligands disrupt the "inactive" circuit and induce connectivity with a second allosteric site. Ligand-contacting residues in helix 7 help mediate the switch between circuits, suggesting new avenues for developing LRH-1-targeted therapeutics. We also elucidate aspects of coregulator signaling, showing that localized, destabilizing fluctuations are induced by inappropriate ligand-coregulator pairings. These studies have uncovered novel features of LRH-1 allostery, and the quantitative approach used to analyze many simulations provides a framework to study allosteric signaling in other receptors.
Keywords: LRH-1; allosteric regulation; ligand regulation; molecular dynamics; nuclear receptor; structure-function.
© 2023 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures





Update of
-
The nuclear receptor LRH-1 discriminates between ligands using distinct allosteric signaling circuits.bioRxiv [Preprint]. 2023 Jan 28:2023.01.27.525934. doi: 10.1101/2023.01.27.525934. bioRxiv. 2023. Update in: Protein Sci. 2023 Oct;32(10):e4754. doi: 10.1002/pro.4754. PMID: 36747705 Free PMC article. Updated. Preprint.
References
-
- Bolado‐Carrancio A, Riancho JA, Sainz J, Rodriguez‐Rey JC. Activation of nuclear receptor NR5A2 increases Glut4 expression and glucose metabolism in muscle cells. Biochem Biophys Res Commun. 2014;446(2):614–619. - PubMed
-
- Case D, Ben‐Shalom IY, Brozell SR, Cerutti DS, Cheatham TE III, Cruzeiro VWD, et al. Amber 2018. San Franscisco: University of California; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

