Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk
- PMID: 37572651
- PMCID: PMC10878405
- DOI: 10.1016/j.medj.2023.07.004
Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk
Abstract
Background: The human endometrium undergoes recurring cycles of growth, differentiation, and breakdown in response to sex hormones. Dysregulation of epithelial-stromal communication during hormone-mediated signaling may be linked to myriad gynecological disorders for which treatments remain inadequate. Here, we describe a completely defined, synthetic extracellular matrix that enables co-culture of human endometrial epithelial and stromal cells in a manner that captures healthy and disease states across a simulated menstrual cycle.
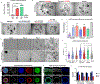
Methods: We parsed cycle-dependent endometrial integrin expression and matrix composition to define candidate cell-matrix interaction cues for inclusion in a polyethylene glycol (PEG)-based hydrogel crosslinked with matrix metalloproteinase-labile peptides. We semi-empirically screened a parameter space of biophysical and molecular features representative of the endometrium to define compositions suitable for hormone-driven expansion and differentiation of epithelial organoids, stromal cells, and co-cultures of the two cell types.
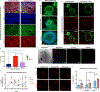
Findings: Each cell type exhibited characteristic morphological and molecular responses to hormone changes when co-encapsulated in hydrogels tuned to a stiffness regime similar to the native tissue and functionalized with a collagen-derived adhesion peptide (GFOGER) and a fibronectin-derived peptide (PHSRN-K-RGD). Analysis of cell-cell crosstalk during interleukin 1B (IL1B)-induced inflammation revealed dysregulation of epithelial proliferation mediated by stromal cells.
Conclusions: Altogether, we demonstrate the development of a fully synthetic matrix to sustain the dynamic changes of the endometrial microenvironment and support its applications to understand menstrual health and endometriotic diseases.
Funding: This work was supported by The John and Karine Begg Foundation, the Manton Foundation, and NIH U01 (EB029132).
Keywords: Foundational research.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.G.G. and V.H.G. have a patent application pending related to the hydrogel system.
Figures




Comment in
-
Engineering the endometrium.Med. 2023 Aug 11;4(8):495-496. doi: 10.1016/j.medj.2023.07.009. Med. 2023. PMID: 37572649
References
-
- Aplin JD (1989). Cellular Biochemistry of the Endometrium. In Biology of the Uterus (Karger Publishers), pp. 89–129. 10.1007/978-1-4684-5589-2_6. - DOI
-
- Loy SLY and S T (2005). Normal cycling Endometrium:Molecular, Cellular, and Histologic Perspectives. In Endometriosis in Clinical Practice, pp. 1–29.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

