A neural circuit for male sexual behavior and reward
- PMID: 37572660
- PMCID: PMC10615179
- DOI: 10.1016/j.cell.2023.07.021
A neural circuit for male sexual behavior and reward
Erratum in
-
A neural circuit for male sexual behavior and reward.Cell. 2024 Jul 11;187(14):3783. doi: 10.1016/j.cell.2024.05.047. Epub 2024 May 29. Cell. 2024. PMID: 38810643 Free PMC article. No abstract available.
Abstract
Male sexual behavior is innate and rewarding. Despite its centrality to reproduction, a molecularly specified neural circuit governing innate male sexual behavior and reward remains to be characterized. We have discovered a developmentally wired neural circuit necessary and sufficient for male mating. This circuit connects chemosensory input to BNSTprTac1 neurons, which innervate POATacr1 neurons that project to centers regulating motor output and reward. Epistasis studies demonstrate that BNSTprTac1 neurons are upstream of POATacr1 neurons, and BNSTprTac1-released substance P following mate recognition potentiates activation of POATacr1 neurons through Tacr1 to initiate mating. Experimental activation of POATacr1 neurons triggers mating, even in sexually satiated males, and it is rewarding, eliciting dopamine release and self-stimulation of these cells. Together, we have uncovered a neural circuit that governs the key aspects of innate male sexual behavior: motor displays, drive, and reward.
Keywords: BNST; LTP; POA; Tac1; Tacr1; aggression; bed nucleus of stria terminalis; brain stimulation reward; dopamine; drive; hypothalamic LTP; hypothalamus; libido; male sexual behavior; mating; motivation; preoptic hypothalamus; reinforcement; reward; social behavior network; substance P.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.L. is a member of the advisory board for Cell; D.W.B., C.-h.O.D., J.R.K., and N.M.S. are named inventors on a patent application that has been filed on the subject matter; A.L. is a son of, and N.M.S. is, a co-founder of Yemaya.
Figures

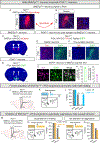





References
-
- Triggs GS (1991). The population ecology of house mice (Mus domesticus) on the Isle of May, Scotland. Journal of Zoology 225, 449–468. 10.1111/j.1469-7998.1991.tb03828.x. - DOI
-
- Bronson FH (1979). The reproductive ecology of the house mouse. Q Rev Biol 54, 265–299. - PubMed
-
- King JA (1956). Sexual behavior of C57BL/10 mice and its relation to early social experience. The Journal of Genetic Psychology 88, 223–229. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

