Dopamine signaling from ganglion cells directs layer-specific angiogenesis in the retina
- PMID: 37572663
- PMCID: PMC10529464
- DOI: 10.1016/j.cub.2023.07.040
Dopamine signaling from ganglion cells directs layer-specific angiogenesis in the retina
Abstract
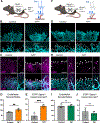
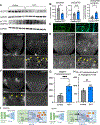
During central nervous system (CNS) development, a precisely patterned vasculature emerges to support CNS function. How neurons control angiogenesis is not well understood. Here, we show that the neuromodulator dopamine restricts vascular development in the retina via temporally limited production by an unexpected neuron subset. Our genetic and pharmacological experiments demonstrate that elevating dopamine levels inhibits tip-cell sprouting and vessel growth, whereas reducing dopamine production by all retina neurons increases growth. Dopamine production by canonical dopaminergic amacrine interneurons is dispensable for these events. Instead, we found that temporally restricted dopamine production by retinal ganglion cells (RGCs) modulates vascular development. RGCs produce dopamine precisely during angiogenic periods. Genetically limiting dopamine production by ganglion cells, but not amacrines, decreases angiogenesis. Conversely, elevating ganglion-cell-derived dopamine production inhibits early vessel growth. These vasculature outcomes occur downstream of vascular endothelial growth factor receptor (VEGFR) activation and Notch-Jagged1 signaling. Jagged1 is increased and subsequently inhibits Notch signaling when ganglion cell dopamine production is reduced. Our findings demonstrate that dopaminergic neural activity from a small neuron subset functions upstream of VEGFR to serve as developmental timing cue that regulates vessel growth.
Keywords: Notch; VEGF; dopamine; dopaminergic amacrine cells; retina; retinal ganglion cells; tyrosine hydroxylase; vasculature.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

