CYP19A1 mediates severe SARS-CoV-2 disease outcome in males
- PMID: 37572667
- PMCID: PMC10518605
- DOI: 10.1016/j.xcrm.2023.101152
CYP19A1 mediates severe SARS-CoV-2 disease outcome in males
Abstract
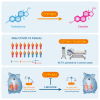
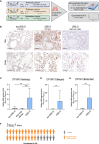
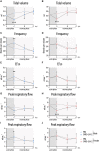
Male sex represents one of the major risk factors for severe COVID-19 outcome. However, underlying mechanisms that mediate sex-dependent disease outcome are as yet unknown. Here, we identify the CYP19A1 gene encoding for the testosterone-to-estradiol metabolizing enzyme CYP19A1 (also known as aromatase) as a host factor that contributes to worsened disease outcome in SARS-CoV-2-infected males. We analyzed exome sequencing data obtained from a human COVID-19 cohort (n = 2,866) using a machine-learning approach and identify a CYP19A1-activity-increasing mutation to be associated with the development of severe disease in men but not women. We further analyzed human autopsy-derived lungs (n = 86) and detect increased pulmonary CYP19A1 expression at the time point of death in men compared with women. In the golden hamster model, we show that SARS-CoV-2 infection causes increased CYP19A1 expression in the lung that is associated with dysregulated plasma sex hormone levels and reduced long-term pulmonary function in males but not females. Treatment of SARS-CoV-2-infected hamsters with a clinically approved CYP19A1 inhibitor (letrozole) improves impaired lung function and supports recovery of imbalanced sex hormones specifically in males. Our study identifies CYP19A1 as a contributor to sex-specific SARS-CoV-2 disease outcome in males. Furthermore, inhibition of CYP19A1 by the clinically approved drug letrozole may furnish a new therapeutic strategy for individualized patient management and treatment.
Keywords: COVID-19; CYP19A1; estradiol; letrozole; lung health; male sex; testosterone.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Method for predicting the course of a viral disease. Inventors: G.G. and S.S.-B. Filing date: 04.30.2021. Pending patent applications: Europe (EP21722231.4), USA (US17995728), Japan (JP2022-566073), China (CN202180031796.5).
Figures





References
-
- Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W., Sanderson C., McKee M., Troeger C., Ong K.L., et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

